1. Background
Obesity is known as a cardiovascular risk factor. The obese people are at a higher risk of harmful fats, which cause sedimentation in the vessels, arterial obstruction, and consequently stenosis. Significant weight gain and obesity along with blood lipid disorders can cause arterial thrombosis and hypertension (1).
Hypertension is one of the most important risk factors for cardiovascular disorders, including coronary artery diseases (2). The progress of coronary artery disease can be controlled by blood pressure control. Hypertension affects one billion people or every three adults around the world. However, more than 50% of patients with hypertension are not aware of their disease status (3). Current medical treatment modalities with standard antihypertensive drugs are not always effective and may not lead to uncontrolled hypertension. Moreover, the side effects of drug treatment may influence compliance with treatment. For this reason, the use of complementary and alternative therapies for these patients has attracted a lot of attention (3). Blood pressure management involves lifestyle modifications, including increased physical activity, weight loss, and dietary changes that can also include nutritional supplements (4).
Mobility is reduced with age increase, which is followed by elevated body weight, fat percentage, and lipid profile. The risk of cardiovascular diseases is high in overweight and obese people, and physical activity is regarded as the completing component of the obesity treatment program and cardiovascular health (5). Regular aerobic exercise improves cardiovascular fitness (6). Numerous studies have shown a negative association between cardiovascular fitness and mortality etiology due to cardiovascular disorders in men and women of all ages (7). Hence, there is a strong logical reason for the importance of physical exercise in lifestyle modification programs to prevent or treat metabolic syndrome and hypertension.
Currently, plant medicines are increasingly being used for the treatment and prevention of different diseases. Since the consumption of plant supplements has fewer side effects than artificial drugs, they can sometimes be an appropriate substitute for drug therapy (8). Garlic is a plant that is widely used for the treatment of aging-related diseases. It has potent antioxidant properties (9), inhibits vascular stenosis and cardiovascular disorders, and reduces blood fat, blood glucose, and blood concentration (10). It has been shown that garlic affects the diameter of vessels and increases the blood flow to arteries and capillaries (10). Concurrent use of exercise and garlic supplement seems to have more beneficial effects on obese patients suffering from hypertension.
2. Objectives
this study investigated the effect of eight weeks of aerobic exercise and garlic extra consumption on the blood pressure, fat percentage, and lipid profile of obese patients with hypertension.
3. Methods
In this quasi-experimental study, 50 obese men with hypertension with a mean age of 53 ± 7.6 years were voluntarily recruited from region three of Tehran, Iran. They had no history of smoking, alcohol use, and other diseases. Obesity was diagnosed by Body Mass Index (BMI) formula, and participants with BMI ≥ 30 were considered obese (Chewi et al, 2018). The participants’ blood pressure was measured by OMRON M2 digital blood pressure monitor with an accuracy of 0.1 mmHg. Systolic blood pressure > 140 mmHg and diastolic blood pressure ≥ 90 mmHg in at least two separate measurements in two different days indicated hypertension (11).
The participants were randomly assigned to five groups, including aerobic exercise, garlic extract, aerobic extract plus garlic extract, placebo, and control. The experimental groups received eight weeks of aerobic exercise and/or garlic extract supplement. This study is taken from a research project approved by Kermanshah University of Medical Sciences (ethical code: IR.KUMS.REC.1398.656) and registered in the Iranian Registry of Clinical Trials (clinical trial code: IRCT 20190918044813N1).
At first, the participants were briefed on the study procedures. They willingly participated in the study after completing the consent forms. It should be noted that all necessary medical and health care were applied during blood taking and exercise training. The garlic extract group received 1 garlic capsule daily (each capsule containing 1000 mg garlic extract, Nature’s Bounty, U.S.). The placebo group also received 1 dextrose capsule (1000 mg) daily (12).
The aerobic exercise protocol included three exercise sessions per week, each session for 35 - 60 min. Each session consisted of 10 min warm-up (jogging and stretching), 20 - 45 min running on a treadmill, and 5 min cooling down at the end. In the first week, exercise was started with a maximum heart rate of 50 - 55, which included 20 min running on treadmill. In the second week, exercise was started with a maximum heart rate of 50 - 55 for 30 min. In the third week, exercise was started with a maximum heart rate of 60 - 70 for 45 min and continued to week six. The exercise intensity reached the maximum heart rate of 70 - 75 for 45 min from week six on and continued to week eight. Maximum heart rate (HR max) for each participant was calculated by the 220-age equation. Exercise intensity was controlled by a pacemaker (Polar, Finland) via heart rate at specific intervals (every five seconds) (13).
At the beginning of the study, 5 cc blood was taken from the patients’ brachial vein after 12 h fasting and kept in the test tubes containing EDTA. Blood samples were immediately transferred to the laboratory for the subsequent measurements. All samples were centrifuged by a centrifuge machine (MSE, England) in the laboratory at 3500 rpm for 15 min at 4°C. Serum was poured in 0.5 mL Eppendorf microtubes by a sampler and stored at -80°C. Forty-eight hours after the last exercise session, blood samples were taken the same as those of the pretest. Triglyceride (TG) level was measured by the enzymatic method using Pars Azmoon diagnostic kit, high-density lipoprotein (HDL) was measured by the enzymatic method using Zist Shimi diagnostic kit, low-density lipoprotein (LDL) was measured by the enzymatic method using Pars Azmoon diagnostic kit, and total cholesterol was measured by Man diagnostic kit.
Data were analyzed by SPSS (version 16) software. Normal distribution of data was analyzed by Kolmogorov-Smirnov test and homogeneity of variances was assessed by Levin’s test. Within-group comparisons were done by the dependent t-test and between-group comparisons were performed by one-way ANOVA and ANCOVA. In the case of statistical significance, the Bonferroni post-hoc test was run. P ≤ 0.05 was considered statistically significant.
4. Results
Data were presented as Mean ± SEM (Standard Error of the Mean). In the pretest, none of the body weight, body fat percentage, blood pressure, total cholesterol level, TG, and serum LDL and HDL variables showed significant differences among the study groups (P ≤ 0.05). However, to eliminate the effect of the covariate variable (pretest scores), the ANCOVA test was used to perform posttest between-group comparisons.
The results of posttest showed mean body weight significantly reduced in the aerobic exercise (t = 12.329, P ≤ 0.001), garlic extract (t = 2.792, P ≤ 0.05), and aerobic exercise plus garlic extract (t = 10.350, P ≤ 0.001) groups, while no significant change was observed in the placebo and control groups (P > 0.05). The results of ANCOVA test also indicated a significant difference in the body weight among the study groups (F = 48.120, P ≤ 0.001) so that a significant weight loss was seen in the aerobic exercise (P ≤ 0.001), garlic extract (P ≤ 0.05), and aerobic exercise plus garlic extract (P ≤ 0.001) groups compared to the control group. Further, aerobic exercise and aerobic exercise plus garlic extract groups showed significantly more body weight loss than the garlic extract group (P ≤ 0.001) (Table 1).
| Variable Group | Weight | Fat (%) | Systolic Blood Pressure | Diastolic Blood Pressure |
|---|---|---|---|---|
| Aerobic exercise | ||||
| Pretest | 106.9 ± 5.5 | 25.5 ± 1.2 | 145.3 ± 1.6 | 93.1 ± 1.9 |
| Posttest | 98 ± 5.1 | 22.4 ± 1.09 | 134.9 ± 1.8 | 88.6 ± 1.2 |
| Garlic extract | ||||
| Pretest | 105.3 ± 5.3 | 23.6 ± 1.3 | 143.5 ± 1.5 | 93.7 ± 5.5 |
| Posttest | 103.2 ± 5.5 | 22.7 ± 1.4 | 135.8 ± 1.7 | 88.9 ± 1.4 |
| Aerobic exercise + garlic extract | ||||
| Pretest | 105.7 ± 5.9 | 23.5 ± 1.06 | 147.1 ± 1.4 | 94.6 ± 1.7 |
| Posttest | 95.1 ± 5.1 | 20.03 ± 0.8 | 133.5 ± 1.8 | 86.2 ± 1.0 |
| Placebo | ||||
| Pretest | 111.2 ± 5.7 | 25.88 ± 1.5 | 146. ± 1.7 | 91.5 ± 1.4 |
| Posttest | 110.9 ± 5.8 | 25.6 ± 1.5 | 147.9 ± 1.8 | 92.3 ± 1.4 |
| Control | ||||
| Pretest | 106.1 ± 6.5 | 24.6 ± 1.36 | 147.6 ± 1.5 | 94.3 ± 1.8 |
| Posttest | 107.6 ± 6.4 | 25.35 ± 1.4 | 148.2 ± 1.7 | 93.0 ± 1.5 |
Moreover, the results of posttest test showed a significant decrease in the mean body fat percentage in the aerobic exercise (t = 7.375, P ≤ 0.001), garlic extract (t = 4.159, P ≤ 0.05), and aerobic exercise plus garlic extract (t = 8.377, P ≤ 0.001) groups, while no significant change was observed in the control and placebo groups (P > 0.05). The results of ANCOVA test also showed a significant difference between groups in mean fat percentage (F = 26.999, P ≤ 0.001) so that a significant decrease was observed in fat percentage in the aerobic exercise group (P ≤ 0.001) and aerobic exercise plus garlic extract group (P ≤ 0.001) compared with the control group. Moreover, aerobic exercise and aerobic exercise plus garlic extract groups indicated a significantly more decrease in the body fat percentage compared with the garlic extract group (P ≤ 0.001) (Table 1).
Furthermore, the results of posttest showed a significant drop in mean systolic and diastolic blood pressures in the aerobic exercise (t = 7.997, P ≤ 0.001 and t = 5.164, P ≤ 0.001, respectively), garlic extract (t = 10.286, P ≤ 0.001 and t = 5.164, P ≤ 0.001, respectively), and aerobic exercise plus garlic extract (t = 5.273, P ≤ 0.001 and t = 6.013, P ≤ 0.001, respectively) groups, while no significant change was found in the control and placebo groups (P > 0.05). In addition, the results of ANCOVA test showed a significant difference among groups in systolic and diastolic blood pressures (F = 14.329, P ≤ 0.001 and F = 22.393, P ≤ 0.001, respectively) so that systolic and diastolic blood pressures reduced significantly in the aerobic exercise (P ≤ 0.05 and 0 ≤ 0.001, respectively), garlic extract (P ≤ 0.05 and 0 ≤ 0.001, respectively), and aerobic exercise plus garlic extract (P ≤ 0.001 and 0 ≤ 0.001, respectively) groups compared with control group (Table 1).
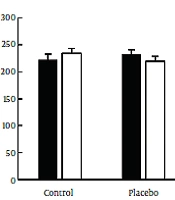
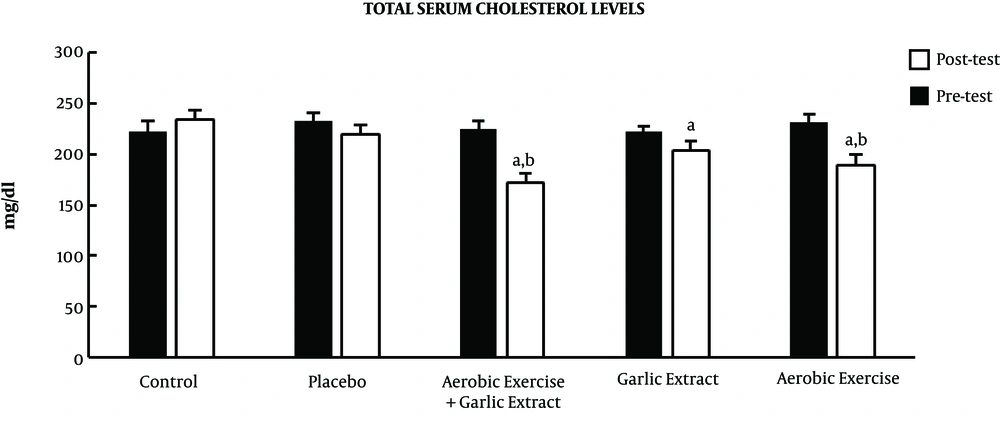
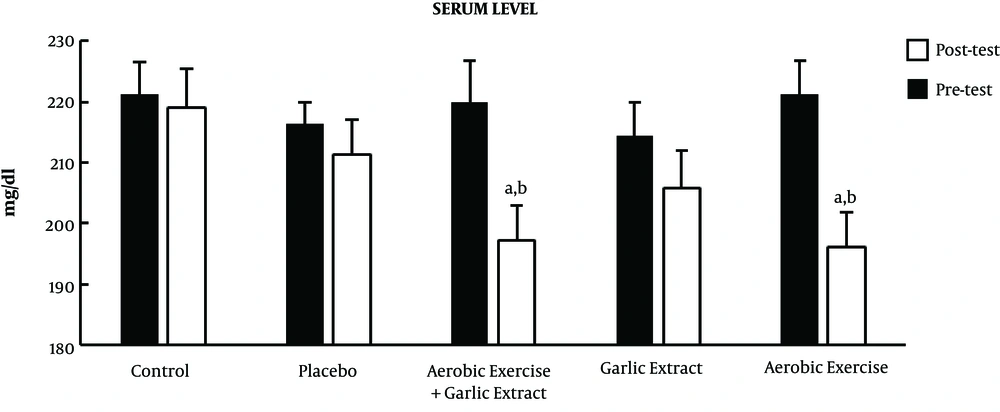
Furthermore, the results of posttest indicated a significant decrease in mean total cholesterol in the aerobic exercise (t = 2.647, P ≤ 0.05), garlic extract (t = 2.987, P ≤ 0.05), and aerobic exercise plus garlic extract (t = 3.943, P ≤ 0.05) groups, while no significant change was observed in the control and placebo groups (P > 0.05). The results of the ANCOVA test also showed a significant difference among groups in the mean total cholesterol level (F = 9.738, P ≤ 0.001) so that total cholesterol level reduced significantly in the aerobic exercise (P ≤ 0.01) and aerobic exercise plus garlic extract (P ≤ 0.001) groups compared to the control group. Further, aerobic exercise and aerobic exercise plus garlic extract groups indicated a significantly higher decrease of total cholesterol compared to the garlic extract group (P ≤ 0.001) (Figure 1).
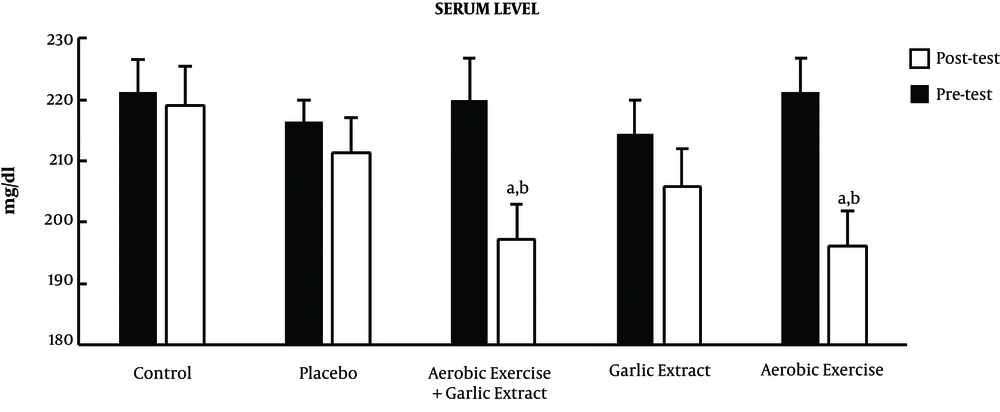
The results of posttest showed a significant drop in the mean TG in the aerobic exercise (t = 3.637, P ≤ 0.01) and aerobic exercise plus garlic extract (t = 2.599, P ≤ 0.05) groups, while there was no significant change in the garlic extract, control, and placebo groups (P > 0.05). Moreover, the results of ANCOVA test indicated a significant difference among groups in the mean TG level (F = 3.747, P ≤ 0.01) so that a significant decrease was found in serum TG level in the aerobic exercise (P ≤ 0.05) and aerobic exercise plus garlic extract (P ≤ 0.05) groups in comparison with the control group (Figure 2).
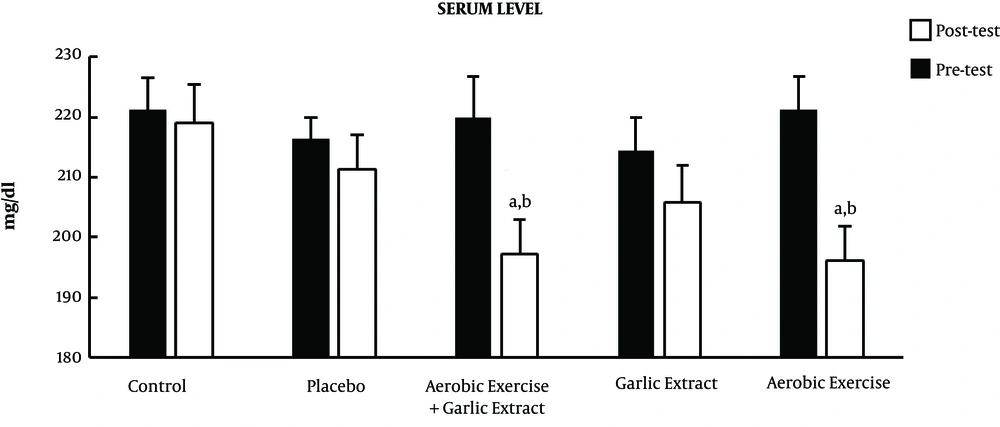
The posttest showed mean LDL significantly decreased in the aerobic exercise (t = 6.726, P ≤ 0.001), garlic extract (t = 19.3, P ≤ 0.001), and aerobic exercise plus garlic extract (t = 11.610, P ≤ 0.001) groups, while no significant change was seen in the control and placebo groups (P > 0.05). In addition, the ANCOVA test revealed a significant difference between groups in serum LDL (F = 44.883, P ≤ 0.001) so that a significant decrease was found in serum LDL in the aerobic exercise (P ≤ 0.001), garlic extract (P ≤ 0.001), and aerobic exercise plus garlic extract (P ≤ 0.001) groups in comparison with control group. Moreover, aerobic exercise plus garlic extract group showed a significantly higher LDL reduction than garlic extract and aerobic exercise groups (P ≤ 0.01) (Figure 3).
As for HDL, the posttest showed a significant increase in the aerobic exercise (t = 6.269, P ≤ 0.001) and aerobic exercise plus garlic extract (t = 6.498, P ≤ 0.001) groups, while no significant change was observed in the garlic extract, control, and placebo groups (P > 0.05). The results of the ANCOVA test also indicated a significant difference in mean serum HDL between groups (F = 25.178, P ≤ 0.001) so that serum HDL level increased significantly in the aerobic exercise (P ≤ 0.001) and aerobic exercise plus garlic extract (P ≤ 0.001) groups compared to control group. Furthermore, aerobic exercise and aerobic exercise plus garlic extract groups indicated a significant increase in HDL level in comparison with the garlic extract group (P ≤ 0.001) (Figure 4).
5. Discussion
This study investigated the effect of eight weeks of aerobic exercise and garlic extract use on the blood pressure, body fat percentage, and lipid profile of obese patients with hypertension. Both aerobic exercise and garlic extract consumption could significantly reduce the systolic and diastolic blood pressures in the patients. However, there was no significant difference between a combination of these two interventions and each intervention alone regarding blood pressure control. Numerous studies have reported reduced blood pressure following regular exercise interventions (14, 15) or garlic supplement use (16). It has been shown that exercise activities moderate the balance between vasodilation and vasoconstriction cytokines such as nitric oxide (17), prostacyclin, and thromboxane (18). A review by Wang in 2015 showed garlic consumption reduced systolic blood pressure by 3.75 mmHg and diastolic blood pressure by 3.39 mmHg in comparison with the control group (19). The possible mechanisms of antihypertensive function of garlic can be associated with the prostaglandin-like effects that reduce peripheral vascular resistance (20). Garlic decreases the prostaglandin E2 and thromboxane B2 (21). Further, Gamma-glutamylcysteines are compounds in the garlic that inhibit angiotensin-converting enzyme (22). Garlic also inhibits the endothelin-1-induced constriction in a dose-dependent manner (23). This study evaluated the mechanisms involved in reducing body weight, fat percentage, as well as lipid profile alterations that can potentially affect blood pressure.
As expected, regular aerobic exercises decreased the body weight and fat percentage of obese patients. Interestingly, regular use of garlic extract caused a significant decrease in body weight. However, the reduction of body fat percentage was not significant in the garlic extract group. These results were in contrast with those of Mahdavi Roshan et al. indicating no change in the BMI following three months of garlic supplement consumption in patients with cardiac disorders (16). This difference can be attributed to the baseline BMI of the participants. The mean BMI of the participants in the above study was 25.5 kg/m2, while that of our participants was over 30. Systolic blood pressure has been reported to be directly associated with BMI in patients with hypertension (16). In line with the findings of the present study, it has been shown that the mean weight decrease of 9 kg in the patients with hypertension is followed by reduced abdominal fat, liver fat, and blood pressure (24).
Another finding of the present study was lipid profile alterations following aerobic exercise and garlic extract use, which can be regarded as another mechanism involved in decreasing blood pressure. Aerobic exercise reduced the total cholesterol, TG, and LDL levels and increased HDL level. However, LDL level reduction was only observed in the garlic extract group. Zakeri et al. reported reduced TG level and increased HDL following 14 days of garlic supplement use (25). Yoon et al. showed that garlic powder use and swimming exercises for four weeks reduced the total cholesterol, TG, and LDL levels and increased HDL level (26). Moreover, Jeon et al. reported garlic powder supplementation together with running on treadmill decreased TG and increased HDL but had no effect on the total cholesterol and LDL levels (27). Yet, differentiating the effects of exercise from garlic supplement is difficult due to the absence of a separate garlic supplement group in the above studies.
Some studies have reported improved lipid profile following exercise activities (28), while some others have shown no significant change in this regard (29, 30). Some researchers believe that lipid profile, especially HDL, is hardly influenced by exercise, which is highly dependent on exercise intensity and amount unless it is followed by a decreased diet or weight loss (20). The primary level of these indices at the beginning of exercise is also an influential factor. On the other hand, more exercise affects the lipid profile of those with higher baseline cholesterol, TG, and LDL and lower HDL level; the higher blood lipids show more tangible changes (31). In this study, the baseline total cholesterol, TG, and LDL of the participants were higher and their HDL level was lower than the normal range, which might be a reason for significant lipid profile changes following aerobic exercise. It has been shown that 1% drop in serum cholesterol level lowers the risk of cardiovascular diseases by 2 - 3% (32). Moreover, in addition to reducing the LDL level, exercise has been reported to induce biochemically beneficial changes in its structure (33).
5.1. Conclusions
Consumption of garlic extract decreases the systolic and diastolic blood pressures in obese patients suffering from hypertension, which is followed by reduced body weight and serum LDL. On the other hand, regular aerobic exercise lowers the body fat percentage and systolic and diastolic blood pressures and induces positive changes in lipid profile. However, the simultaneous use of aerobic exercise and garlic extract has no additional effects on the blood pressure control although it influences the body composition more.