1. Background
In-vitro fertilization (IVF) for human oocyte for the first time was administered by Steptoe and Edwards in 1978 (1). The success of this approach has extended with new technological innovations, so that risk of being pregnant after a cycle of hormonal stimulation has raised more than 25 - 30% (2). A fundamental step of IVF treatment is recovery of oocytes from the ovary. The procedure of transvaginal oocyte retrieval (TVOR) also called oocyte retrieval (OCR), is an important part of IVF treatment. This procedure requires a relatively long operative time and analgesia (3), though in comparison with laparoscopic approach it is less invasive (4). There are various pain relief techniques used for transvaginal oocyte retrieval which has been proven in a survey to be used as follows: Monitored anesthesia care (MAC) in 46% of the centers, General anesthesia (GA) in 28%, regional anesthesia with sedation in 12% and a cocktail regime in the 14% other centers (5). These techniques are applied in order to reach harmless and efficient optimal surgical environments which facilitate analgesia and quicken the post-operative recovery. But, there are some concerns about the possible effects that drugs applied in these techniques can impose on reproductive outcome (6). Analgesic/sedative agents have shown to adversely affect the oocyte maturation and fertilization in animal studies (7).
The oocyte retrieval can be a stressful experience due to the penetration of vaginal mucosa and the ovarian capsule, though various factors modify this stress. It has been suggested that the optimal pain relief procedure would embrace the flexibility to respond to the altering needs of oocyte recovery in women. Patient-controlled analgesia (PCA) using an individualized approach allows women to have some amounts of control over administering drug which due to more satisfaction of patients (8). Some advantages are shown for conscious sedation such as maintaining patient co-operation and performing the procedure conveniently without anaesthetist in the outpatient setting. This method is used frequently for providing analgesia/anesthesia during transvaginal oocyte retrieval (9), as the statistics shows 95% usage in IVF centers of USA (10), and 84% usage in IVF clinics of UK (11). In the other hand, approximately 50% of clinics in Germany and 16% of UK clinics use GA for IVF procedures (12).
Electro-acupuncture has reported to be a suitable alternative to conventional anesthesia during egg collection for IVF (6). Administration of para cervical block (PCB) is already used combined with opioids, sedatives, hypnotics, and also acupuncture with or without premedication during oocytes retrieval via transvaginal ultrasound in some researches (3, 4, 13). Transvaginal puncture for oocyte retrieval is terrifying for many patients because this method seems stressful and painful for them, and they prefer using MAC or pain relief and some favour GA.
The high doses of diverse local anesthetics have undesirable effects on fertilization and embryonal development (14). Anaesthetic agents have also been detected in follicular fluid and may interfere with fertilization. However, regarding the fact that oocytes are washed after retrieval and significantly lower concentrations are attained clinically, using local anesthetics seems to have limited clinical reliability and no negative effects have been shown on fertilization or implantation rates in human trials (15). In mouse embryos under exact tests, the nitrous oxide harmfully affected DNA synthesis by decreasing the number of embryos. Conversely, in human studies the effect of nitrous oxide-isoflurane anesthesia on IVF pregnancy was not significant. Only sporadic findings show that using of general anesthesia (GA), especially with nitrous oxide, for oocyte retrieval can negatively affect the outcomes of IVF.
Opioids like fentanyl and remifentanil have not appeared to affect reproductive success (16). Furthermore, the application of midazolam and ketamine during assisted reproduction is reported as safe drugs (17). Recently, new findings are supporting the use of propofol as a safe alternative assisted reproduction (18).
The present study investigated the effects of GA vs. MAC methods on oocyte retrieval and IVF outcome among women under IVF programs.
2. Objectives
The present study investigated the effects of GA vs. MAC methods on oocyte retrieval and IVF outcome among women under IVF programs.
3. Methods
The statistical population of the study included all women who applied for IVF services in 2009 and referred to the centers providing these services in the country. Of these people, 180 people were collected by random sampling method available as a research sample. The inclusion criteria were as follows: All samples ranged in age from 30 to 45 years, had infertility due to uterine and ovarian disorders, had no specific or severe disease at the time of the study, and were not regular or recreational drug users.
Ultrasound-guided follicular aspiration was scheduled to apply for 180 patients who were recruited for this research. 90 women received MAC, while 90 patients were in GA group. All patients were fasting for 8 hours. MAC group patients were merely given intravenous injections of ketamine and midazolam, with an individualized dose based on the clinical necessities and patients’ comfort. In the GA group, propofol (4 mg/kg) and fentanyl (1 μg/kg/min) induced the anesthesia. In order to maintain the anesthesia, patients took fentanyl (0.10 – 0.20 μg/kg/min) in combination with isoflurane (0.5 vol % end tidal) or diazepam (1.5 – 2.5 mg/kg/h). Samples were instructed to avoid smoking (including painkillers, contraceptives, hormones, nervous system stimulants, and the like) for three days prior to the research protocol.
Ovarian hyper-stimulation was achieved for all patients, after a protocol aiming pituitary down-regulation, which started at the midluteal cycle of the prior phase, by means of triptorelinacetat. Analogue ovarian stimulation was achieved by human menopausal gonadotrophin 100 to 350 IU, after 10 days of luteal suppression by gonadotrophin releasing hormone. Follicular development was supervised by ultrasonography and serum estradiol assessments. 40 hours later oocyte recovery was performed in participants by transvaginal follicular aspiration. Period of this process was between 15 - 18 minutes. Then, culturing the recovered oocytes were placed in cell culture dishes in Hams F-10 medium, which was accompanied by heat inactivated patient serum at 36 - 37°C for 3 - 4 hours already to fertilization. At the first 24 hours of incubation, 15 percent serum was applied and the second 24 hours’ incubation period 30 percent was used in Harms, F 12 medium.
100,000 to 120,000 motile spermatozoa were gathered from male spouse and after centrifugation were used to inseminate the oocytes (three fertilized oocytes were incubated for 24 hours more). Luteal support with 75 mg progesterone was provided for patients by two prescribed doses per day which started 24 hours before transfering embryo and persistent up to 12th gestation week. The factors which were selected for comparison of MAC and GA impacts were as follows: the number of fertilized and transferred oocytes, overall number of retrieved oocytes, and the final pregnancy rate.
The consent forms were filled and signed by the all patients. The ethical mandate of study was approved by the Research Ethics Committee of Sarem Hospital. Statistical analysis was administered using two tests of the Mann-Whitney-U and Fisher’s exact test in their appropriate places. The level of significant was set at P < 0.05; the descriptive data are indicated as means and standard deviations (SD).
4. Results
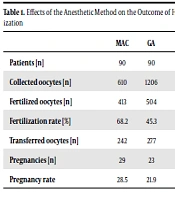
The analysis of participants was first distinguished according to age, primary IVF indications, the technique used for ovarian stimulation, and the level of serum estradiol on the day that HCG was administered. Using GA a number of 1206 oocytes was gathered (Table 1) with a mean number of 11.25 ± 4.29 per patient (Table 2). This was higher in the MAC group: 610 oocytes in overall numbers and a mean of 7.03 ± 4.14 oocytes for each patient (P < 0.001). However, there was no significant difference between groups regarding the number of fertilized oocytes (4.52 ± 3.18 with GA vs. 4.15 ± 3.02 with MAC). Furthermore, the differences regarding the number of pregnancies or the number of transferred oocytes were not significant.
| MAC | GA | P value | |
|---|---|---|---|
| Patients [n] | 90 | 90 | Not significant |
| Collected oocytes [n] | 610 | 1206 | 0.001 |
| Fertilized oocytes [n] | 413 | 504 | Not significant |
| Fertilization rate [%] | 68.2 | 45.3 | - |
| Transferred oocytes [n] | 242 | 277 | Not significant |
| Pregnancies [n] | 29 | 23 | Not significant |
| Pregnancy rate | 28.5 | 21.9 | - |
| MAC | GA | P value | |
|---|---|---|---|
| No. of patients | 90 | 90 | Not significant |
| Mean no. of collected oocytes | 7.03 ± 3.84 | 11.25 ± 4.29 | 0.001 |
| Mean no. of fertilized oocytes | 4.15 ± 2.68 | 4.25 ± 2.76 | Not significant |
| Mean no. of transferred oocytes | 2.36 ± 0.87 | 2.39 ± 0.90 | Not significant |
The data in Table 3 shows that most causes of infertility in subjects were unexplained, after that male problems stands, then ovulatory and tubal problems exist.
| Variables | Values |
|---|---|
| Age (y), mean ± SD | 31.8 ± 5.2 |
| Weight (kg), mean ± SD | 70.3 ± 8.4 |
| Height (m), mean ± SD | 1.64 ± 0.09 |
| BMI (kg/m2), mean ± SD | 26.13 ± 3.1 |
| Smoking, No. (%) | 14 (12.6) |
| Age of menarche (y), mean ± SD | 12.86 ± 1.5 |
| Infertility, N | |
| Primary | 63 |
| Secondary | 27 |
| Cause of infertility, N | |
| Unexplained | 31 |
| Male | 27 |
| Ovulatory | 23 |
| Tubal | 9 |
As can be seen from the findings in Table 1, there were significant differences in fertility rate and pregnancy rate in terms of number of eggs collected.
Based on the findings in Table 2, the two groups differed significantly in terms of the average number of eggs collected.
5. Discussion
In this study, we investigated the question of whether general anesthesia with fentanyl-propofol without nitric oxide could be a viable option for the ketamine-midazolam-supervised procedure to be used in IVF if general anesthesia is required. General anesthesia or monitored anesthesia care (MAC) using anesthetic agents have been detected in follicular fluid. The induction agents thiopental and thiamylal and the levels of follicular fluid in them, has been investigated (16). Recently a few researches offered pharmakokinetic data explaining the period of both (11). Wikland et al. (19) tried to recognize lidocaine in follicular fluid for applying paracervical blocks, while, measureable amounts of propofol or fentanyl and midazolam are demonstrated through transvaginal oocyte retrieval (19-23). However, general anesthetics are identified in follicular fluid, thus, there is a worry about these possible damage that these drugs can bring to the oocyte and follicular structure which might inhibit the IVF success .(24)
Gin and Fhkam stated that the continued exposure of oocytes to the mixture of anesthetic ingredients could cause fatal consequences for the oocyte (25). Guasch et al. detailed that declined fertilizing rates of ova gathered following extended exposure about 50% nitrous oxide and almost 1% isoflurane or enflurane anesthesia (9). Analogous findings were described by De Suter et al. (23).
The current study showed that the number of retrieved oocytes were considerably greater with fentanyl-propofol based GA than with MAC. Considering these results, GA shortly appeared to increase the oocyte retrieval success rate, while these results are most conceivably illuminated by the enhanced ease for the patient throughout the process of transvaginal puncture. Nevertheless, with GA, the more retrieved oocytes number had an inferior fertilization rate, subsequently inducing nearly similar fertilized oocytes rates for each patient. Meanwhile the factors with eminent impact on the number of fertilized oocytes were similar in two group; for instance: ovarian stimulation protocol, age, needle gauge, response of estradiol, and medium of culturing). Therefore, this explanation can clarify the results: Fertilization rates were different between the groups maybe because the oocyte milieu affecting oocyte quality were different (26-28).
Our findings regarding IVF outcomes showed comparable data to other studies with ketamine-midazolam-supervised procedure. However, care must be taken on administered doses of propofol, as a negative impact of high doses propofol on oocyte quality and fertilization have been shown in initial and experimental. On the other hand, doses used in our study were significantly lower than those used in studies related to the negative effect of propofol on the reproductive outcome. Specifically, in these studies propofol was administered as a dose of 2.5 mg/kg followed by continuous infusion of 200 microgram/kg/min or 500 microgram/kg/min, respectively, and was associated with a significantly higher rate of abnormal fertilization (29). The experimental data showing that the toxic effect of propofol on the ability of oocytes to be fertilized is dose-dependent (30).
Jensen et al. analyzed fertilization rates of mature oocytes gathered with either GA or intravenous MAC in an IVF context (31). They demonstrated that the difference between the first and last gathered oocyte was not significant, however showed a tendency to lower fertilization rates with elongated exposure to anesthetic drugs, while it was further manifested for GA than for intravenous MAC. Furthermore, GA may additionally defeat the secretion of gonadotrophin-releasing hormone and gonadotrophin with a present spur of prolactin release (29). It seems feasible that the declined level of gonadotrophin could negatively impact the following luteal phase and development of endometrial part. Nonetheless, Forman et al. (32) in their study showed that greater prolactin levels were not able to modify fertilization rates.
Conversely, Quigley et al. (1982) studied the correlation between follicular size and the oocyte recovery and fertilization rates (33). Their findings indicated that the oocytes obtained from smaller follicles, presented a considerably lesser fertilization rate. The lower fertilization rate with GA comparing the MAC in the present study, can be described by Quigley et al explanations.
In summary, our findings demonstrated that nitrous oxide free application of fentanyl-propofol based GA can be appropriate substitute to MAC and can be suggested for IVF oocyte retrieval, when GA is demanded.