1. Background
Nanotechnology research and development is an expanding discipline, and the global output of new nanoparticles is rising. The NPs have application in various fields, including biomedicine, diseases diagnosis, drug, agriculture, food industry, antimicrobial therapy, cosmetics, toothpastes, shampoos, paints, food supplements, water treatment, etc (1). Despite the benefits, NPs have led to potential toxicities in both humans and animals. Among the nanomaterials, silver nanoparticles (AgNPs) has gained interest because of their distinctive properties, such as chemical stability, antibacterial activity, antifungal, antiviral, and anti-inflammatory characteristics (2). In recent years, there has been a surge in the production and use of silver nanoparticles (AgNPs), which will account for more than half of all global nanomaterial products by 2024 (3). Passing through cell membranes and interacting with biomolecules, silver nanoparticles induce DNA and protein damage and cell modification (4). Toxicity induced by silver nanoparticles (AgNPs) and the role that oxidative stress plays in this process were demonstrated in human cells (5). Medicinal plants and natural products, on the other hand, have been utilized as traditional therapies for a variety of ailments for centuries (6). The phytochemical elements of medicinal plants are responsible for the majority of their pharmacological effects (7). In the western states of Iran, a plant of Snapdragon with the scientific name of Scrophularia striata Boiss has traditional medical usage. The different extracts of this plant with the local name of Teshneh Dary are used in treatment of many infections. Scrophularia striata's wound-healing properties (8), antibacterial effects (9), anti-inflammatory activities and anti-pain (10), anti-cancer properties (11), antioxidation (12), ranging reduced edema, infiltration, T-cells proliferation and their nitric oxide production anti-activity have been studied (13).
2. Objectives
Based on a review of the unique characteristics of S. striata and the fact that no research has been done on the plant's protective properties against AgNP toxicity, the goal of this study is to assess the hemathological modulatory and protective effects of a hydroalcoholic extract of S. striata in the rat model due to AgNP hepatotoxicity as a traditional ethnomedical method.
3. Methods
3.1. Plant Sample Collection
In the spring, S. striata’s fresh leaves were harvested in the province of Kermanshah and its environs. A botanist validated the plant's specifications with plant code No 42801, Kuh.
3.2. Preparation of the Extract
The leaves of S. striata were washed, chopped, and air-dried at room temperature in the shade before being processed into powder with a laboratory grinder. To create hydroalcoholic extract, the powder was extracted three times for 48 h using a 70/30 (V/V) combination of ethanol and water. We filtered the extract with Kartuosh filter paper. At a temperature of 40°C, we used a rotary evaporator to evaporate and concentrate the solvent. The amount of dry extract obtained was 0.22 mg. The dried extract was kept at 4°C until further usage. In this study, the correct doses of the extract were made by mixing the dried extract with normal saline (14, 15).
3.3. Preparation of Nanoparticles
The Pishgaman Iranian Nanomaterial Company provided a silver nanoparticle solution with a concentration of 4000 p. The AgNPs have a diameter of 5 to 8 nm. UV/Vis spectrophotometry (Biotek Epoch, USA) and inductively coupled plasma optical emission spectrometry (ICP-OES, Cambridge, UK) were used to filter the AgNPs, and scanning electron microscopy (SEM) and transmission electron microscopy (TEM) techniques were used to confirm their original physical and chemical properties. Pishgaman Iran Nanomaterials Company was in charge of standards. Silver nanoparticles were prepared and characterized by swirling 100 mM silver nitrate into a 1 percent (w/v) tannic acid solution (pH adjusted to 8 by adding 150 mM potassium carbonate) of polyvinyl pyrrolidone (PVP). The solution turned a pale-yellow color (AgNPs). Following the manufacturer's directions, we diluted the original solution to 20 units to reach the toxicity concentration (200 ppm). After that, the animals were gavaged every day for 30 days (16, 17).
3.4. Experimental Animals
Based on the inclusion and exclusion criteria, thirty adult Wistar rats were bought from the Animal Care Unit of the Faculty of Veterinary Medicine at Razi University in Kermanshah, Iran. Prior to beginning the investigation, it was crucial to identify these variables. The following are the requirements for inclusion: Male Wistar rats aged 8 to 10 weeks, weighing 200 to 210 g, with typical behavior and activity. The following are the conditions for being excluded: female Wistar rats, male Wistar rats who were sick or frail, and rats that had been used in any previous inquiry were excluded from the study. Six rats per cage were housed in stainless steel cages under regulated environmental conditions of temperature (22 ± 2°C), relative humidity (55 ± 5%), and illumination (12-h light/12-h dark cycle). All of the rats were fed regular lab pellets and had access to clean water at all times.
3.5. Experimental Design
This experimental research study was carried out between June 2019 and August 2019 at the biomedical laboratory of the Department of Basic Science sector of immunohematology at the Faculty of Veterinary Medicine, Razi University. After that, the rats were divided into five groups, each with six animals. The normal control group consisted of rats that were given normal saline orally and regularly. Animals in group 2 were only given AgNPs (200 ppm) orally and on a regular basis. The hydroalcoholic extract of S. striata was given to rats in groups 3, 4, and 5 by oral gavage at dosages of 20, 60, and 180 mg/kg body weight, respectively, with AgNPs. The dosages of AgNPs and S. striata extracts used in this investigation were calculated using previous publications and our own research (18, 19). The investigation lasted 30 days. In accordance with the National Institutes of Health Guidelines for the care and use of laboratory animals in biomedical research, the Animal Ethics Committee at Razi University gave its approval for the study to be conducted (Animal Ethical Approval Number: 396-2-038).
3.6. Animals Euthanize
At the end of the treatment period of thirty days, the animals were euthanized by giving them an intraperitoneal injection of 1 mg/kg Xylazine HCl (Xylazine 2%; Alfasan) and 0.5 mg/kg Ketamine HCl. This combination caused the animals to die quickly (Ketamine 5 percent; Trittau, Germany) (20).
3.7. Sample Collection
The Whole blood was extracted from the heart and to a standard laboratory tubes with anticoagulation (EDTA) and one minute were shaked to prevent possible blood clotting. The hematology factors including, NE% (percent of neutrophils), WBC (white blood cell count), MO% (percent of monocytes), LY% (percent of lymphocytes), EO% (percent of eosinophils), RBC (red blood cell count), BA% (percent of basophils), HCT (hematocrit), Hb (hemoglobin concentration), MCH (mean corpuscular hemoglobin), MCV (mean corpuscular volume), MCHC (mean corpuscular hemoglobin concentration), PLT (platelet count), and MPV (mean platelet volume) were analyzed using a blood cell counter auto analyzer (Sysmex KX-21N Technology, Japan) (21, 22).
3.8. Statistical Analysis
The acquired data were analyzed with SPSS software for Windows (Version 21, Chicago, IL, USA), using one-way analysis of variance (ANOVA) and Tukey's HSD post-hoc test. The significance level between the groups was fixed at P < 0.05 and the findings were presented as mean ± SEM.
4. Results
4.1. Granulocyte & a-granulocyte Parameters
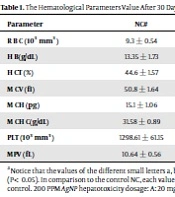
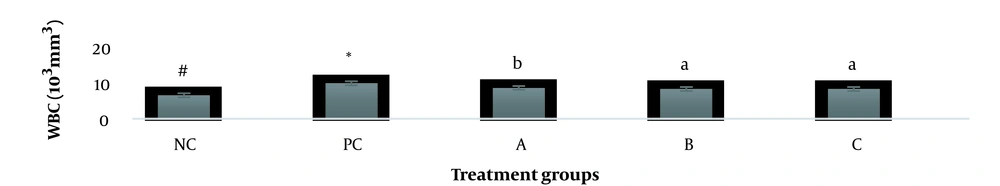
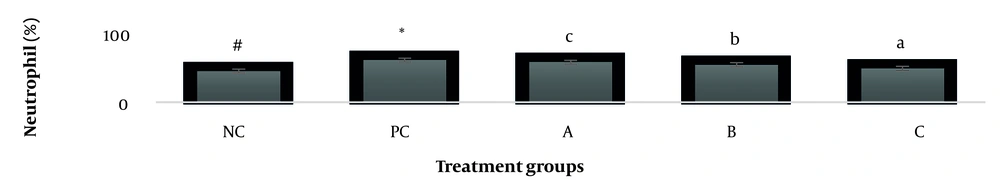
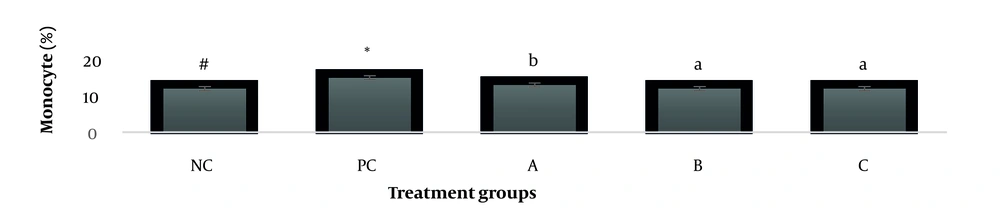
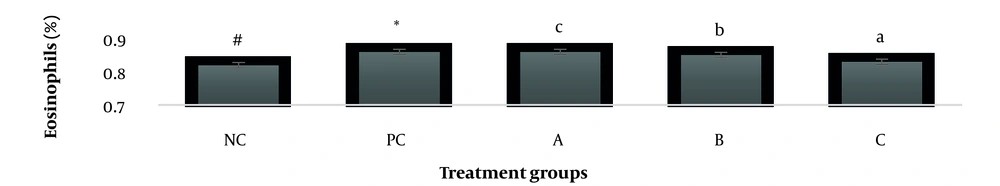
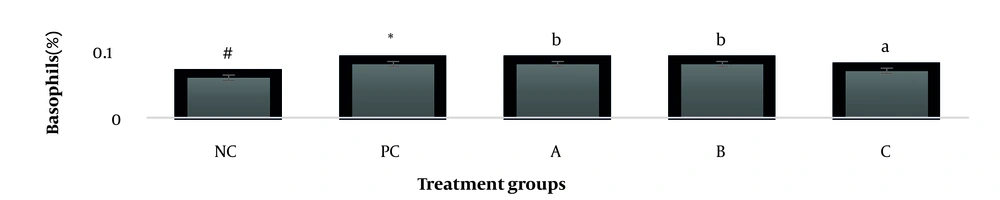
The results showed, in all studied factors (granulocyte & agranulocyte parameters) administration of Ag nanoparticles and induction hepatotoxicity with a dose of 200 ppm, changed in the positive control group compared to the negative control group. It was also demonstrated that administration of S. striata hydroalcoholic extract in treatment groups (20, 60, and 180 mg/kg) together with AgNPs 200 ppm (Dose of hepatotoxicity) in a dose-dependent, especially group 4 (60 mg/kg) and group 5 (180 mg/kg) had hematomodulations of granulocyte and a-granulocyte parameters in comparison with the positive control groups and could modulate the cytotoxicity effects of silver nanoparticles in rats. Consequently, extract dosage at concentrations 20 mg/kg, 60 mg/kg, and 180 mg/kg (groups 3, 4, and 5) caused the levels of WBC, Neutrophils, Lymphocyte, monocyte, eosinophils, and basophils to get close to the negative control (P < 0.05), (Figures 1-6). In order, the highest effect of the extract on indices WBC (8.3 ± 0.4, 8.3 ± 0.37) was related to the concentrations of 60&180 mg/kg (Figure 1). The highest dosage of the extract of S. striata at concentrations of 180 mg/kg (group 5) showed hematomodulation of Neutrophils (49 ± 0.77) and the highest dosage of the extract at concentrations of 60&180 mg/kg (groups 4 & 5) showed hematomodulation of Lymphocyte (33 ± 0.94 & 33 ± 1.23) levels compared with the positive and negative control (P < 0.05), (Figures 2 and 3). The highest effect of the extract on indices monocyte (12 ± 0.65 & 12 ± 0.68), was related to the concentration of 60 & 180 mg/kg (groups 4 & 5) (Figure 4). The results showed that hematomodulated levels of eosinophils (0.83 ± 0.01) in 5group & basophils (0.08 ± 0.05, 0.08 ± 0.01 & 0.07 ± 0.61) in 3, 4 & 5 groups in comparison with the positive and negative control (Figures 5 and 6), (P < 0.05).
Basophil parameter after 30 days administration the AgNP & Scrophularia striata extract with significantly different the three treatment groups in comparison with the control groups (P < 0.05). Notice that the values of the different small letters a, b, and c indicate a significant difference between the examined groups and the PC* and NC# groups, respectively (P < 0.05). In comparison to the control NC, each value is presented as the mean ± SD of the three replications: PC stands for positive control, and PC stands for negative control. 200 PPM AgNP hepatotoxicity dosage: A: 20 mg/kg, B: 60 mg/kg, C: 180 mg/kg.
4.2. General Parameters
The evaluation of the data revealed that the treatment of 200 ppm AgNPs altered the range of general blood parameters in rats (RBC, Hb, HCT, MCV, MCH, MCHC, PLT, and MPV) in both the positive and negative control groups. In addition to this, it was demonstrated that the administration of S. striata hydroalcoholic extract in treatment groups (20, 60, and 180 mg/kg) along with AgNPs 200 ppm, especially group 5 (180 mg/kg) and group 4 (60 mg/kg) had hematomodulations on blood general parameters in comparison with the positive control groups and could modulate the cytotoxicity effects of silver nanoparticles (P < 0.05), (Table 1). In order, the highest effect of the extract on indices RBC (8.4 ± 0.69), was related to the concentrations of 180 mg/kg. The maximum dose of S. striata extract at concentrations of 180 mg/kg (group 5) exhibited hematomodulation of Hb (11.15 ± 1.39) and HCT (38.8 ± 0.43) levels relative to the positive and negative control (P < 0.05). The effect of the extract on indices MCV (46.3 ± 1.01 & 46.93 ± 0.63) was related to the concentration of 60 & 180 mg/kg. The highest effect of the extract on indices MCH (13.2 ± 0.57 & 13.2 ± 0.99) & MCHC (30.4 ± 0.6), was related to the concentration of 60 & 180 mg/kg. The findings demonstrated hematomodulated levels of PLT (1075.3 ± 14.1 & 1075.73 ± 9.98) in groups 4 and 5 and MPV (10.88 ± 0.86) in group 5 relative to the positive and negative control (P < 0.05). According to collected results, treatment with S. striata extract substantially dose-dependently regulated hematomodulation levels of the aforementioned parameters compared to AgNPs-treated rats (P < 0.05) (Table 1).
| Parameter | NC# | PC* | A | B | C |
|---|---|---|---|---|---|
| R B C (103 mm3) | 9.3 ± 0.54 | 7.5 ± 0.43 | 7.7 ± 0.56 c | 8.03 ± 0.48 b | 8.4 ± 0.69 a |
| H B(g/dL) | 13.35 ± 1.73 | 10.5 ± 0.79 | 10.75 ± 1.2 c | 10.95 ± 0.99 b | 11.15 ± 1.39 a |
| H CT (%) | 44.6 ± 1.57 | 35.2 ± 0.87 | 37.4 ± 0.84 c | 38.5 ± 0.76 b | 38.8 ± 0.43 a |
| M CV (fL) | 50.8 ± 1.64 | 44.95 ± 1.92 | 45.95 ± 0.97 c | 46.3 ± 1.01 a | 46.93 ± 0.63 b |
| M CH (pg) | 15.1 ± 1.06 | 12.5 ± 0.99 | 12.8 ± 1.6 b | 13.2 ± 0.57 a | 13.2 ± 0.99 a |
| M CH C(g/dL) | 31.58 ± 0.89 | 29.1 ± 1.17 | 30.1 ± 0.83 b | 30.4 ± 0.6 a | 30.13 ± 0.91 b |
| PLT (103 mm3) | 1298.61 ± 61.15 | 1050 ± 96.25 | 915.5 ± 37.65 b | 1075.3 ± 14.1 a | 1075.73 ± 9.98 a |
| MPV (fL) | 10.64 ± 0.56 | 11.64 ± 0.19 | 11.26 ± 0.21 b | 11.21 ± 0.44 b | 10.88 ± 0.86 a |
aNotice that the values of the different small letters a, b, and c indicate a significant difference between the examined groups and the PC* and NC# groups, respectively (P < 0.05). In comparison to the control NC, each value is presented as the mean ± SD of the three replications: PC stands for positive control, and PC stands for negative control. 200 PPM AgNP hepatotoxicity dosage: A: 20 mg/kg, B: 60 mg/kg, C: 180 mg/kg.
5. Discussion
Fresh and green components of S. striata have been shown to be employed in traditional medicine studies. S. striata contains anti-inflammatory flavonoid components (iridoids, phenolic acids, quercetin, isorhamnetin-3-O-rutinoside, cinnamic acid, polyphenolic acid, nepitrin, chlorogenic acid, phenylpropanoid glycoside, and other glycoterpenoids) according to phytochemical analysis (10, 23). Therefore, in this study, the possible hematomodulation of S. striata extract against AgNPs-induced hepatoxicity was investigated. The CBC test, which examines the morphological characteristics and number of different types of blood cells (Red blood cells, Leukocytes, and Platelets) per cubic millimeter of blood, is one of the most common tests and provides valuable information about different types of blood cell disorders, such as anemia (24).
The WBC disorders are mostly seen as proliferative, leukopenia, and cell dysfunction (25). Experimental studies have shown that long-term oral administration of Ag-NP's toxic dose in mice (28 - 30 days), increases spleen size and weight, total number of T, B, Mon and Nut cells (26), serum levels of IgM, IgG, proinflammatory cytokines and inflammation such as IL-1β, TNF-α, IL- 6, IL-4, IL-10, IL-12, and TGF-β (27), granulocytes and a-granulocytes blood (28). An increase in the numbers of white blood cells, may indicate an antigen response or dysfunction of the bone marrow, liver, and spleen (29). Furthermore, earlier studies have shown that the hazardous potential of Ag- NPs affects some of the primary processes in inflammation (oxygen reactive species (ROS) formation and complement system, nitric oxide (NO) and COX-2 pathways) (30).
In the present study, the levels of granulocyte and a-granulocyte parameters (WBC, Neutrophil, lymphocyte, monocyte, eosinophils, and basophils) were increased in the positive control group due to the hepatotoxicity. The above results were consistent with the studies of Lee et al. (31), Dosoky et al. (32) and Pinzaru et al. (33) The several studies have demonstrated the antioxidant and immunomodulatory properties of S. striata extract by using its anti-inflammatory mechanisms and with the help of Th2s that suppressed IL-10, IL-13, TGF-β, IL- 1β, TNF-α, NO and PGE2, reduced inflammation and thus the amount of white blood cells modulated (18, 34, 35). The hemathomo modulatory effects of S. striata extract with AgNPs 200 ppm were observed in treatment groups A, B, and C at the levels of granulocytes and a-granulocytes blood parameters, which is consistent with the findings of Abedi et al. (36), Rostami et al. (37), and Mahdavi et al. (38) studies on hemathomo modulatory activity and growth performance. Therefore, based on the chemical analysis of S. striata, the hemathomodulation properties of the plant extract are related to the presence of several important anti-inflammatory compounds (phenolic acids, flavonoids, iridoids and phenylpropanoid glycosides).
Determination of RBC and the whole blood HGB level is a suitable parameter to assess the ability of blood to deliver oxygen to tissues and organs and to transport CO2 to the lungs. The HCT indicates the percentage of red blood cells in total blood volume and decreases as the number of red blood cells decreases. The MCV shows red blood cell volume and has diagnostic value in anemia. The MCH index shows the average amount of hemoglobin in red blood cells and has diagnostic value in anemia. The MCHC indicates the average concentration of hemoglobin in red blood cells. Also, obtained results on CBC in this study showed an alteration in quantity of red blood cells parameters. Several mechanisms have been described for changes in blood parameters after nanoparticle consumption. For example, by their toxicological properties, Nanoparticles disrupt hemoglobin synthesis by increasing ROS, free radicals, oxidative stress (39, 40), thus there'll be liver damage, low metabolic activity, negative effects on bio kinetics (41), lipid peroxidation increase (42), and bone marrow suppression during erythrocyte maturation, in addition to disrupting RBC membrane integrity and erythrocyte hemolysis (43, 44). Several authors have suggested that silver nanoparticles link to sulfur and phosphorus contained in biomolecules like DNA or other biological compounds, thus causing cell toxicity (45). Increased RBC degradation and decreased RBC count due to iron, cobalamin, or folic acid deficiency have been reported with oral administration and intraperitoneal injection of Ag NP (46). The decrease in the red blood cell and HGB may have been resulted from the suppression of circulating erythropoietin hormone (a glycoprotein which stimulates the process of erythropoiesis) (47, 48). Inflammation of the gut and impaired absorption of iron, resulting in the production of inflammatory cytokines such as IL-6, which increase the production of hepcidin. With its inhibitory effect on ferroprotein-1, Hepsidine reduces the release of iron in the intestinal epithelial cells into the plasma, which in turn reduces the body's ferritin level and prevents the transformation of iron to pronormoblasts (49).
The significant decreases in the levels of RBC, HGB, HCT MCH, MCHC and MCV with the dosage of 200PPM Ag-NP for 30 days reflects the hematotoxic effects of Ag-NP which could be attributed to the impaired absorption of iron, increase in the production of hepcidin & free radicals that compromise the integrity of the membrane. In this study, RBC and HGB levels increased and afterwards increase of HCT, MCV, MCH and MCHC levels were observed in three treatments groups (A, B & C) in comparison with the PC groups. The results of the present study correlate with several reports on the hematotoxic of AgNPs (50, 51), and this results were consistent with the Rostami et al., and Mahdavi et al. on hemathomomodulatory effects of S. striata studies (37, 38).
The PLT shows the quantity of platelet cells in the blood, and too much or too little leads to blood clotting disease and MPV shows the volume of platelets and increased or decreased platelet volume provides information about bone marrow platelet production (52). Also, the significant decreases in the levels of PLT counts and MPV (In treatment groups A, B & C) under the effect of 200PPM Ag-NP for 30 days reflects the hematotoxic effects of Ag-NP which could be attributed to the production of free radicals that compromise the integrity of the membrane. The results show the modulatory effects of S. striata extract on PLT counts and MPV, in three treatments groups (A, B & C) in comparison with the PC groups. Therefore, in the analysis of the data, the hematomodulation effects of S. striata are probably due to the presence of antioxidant compounds such as phenolic acids, flavonols and flavonoids (14, 53), which reduces the free radical damage of silver nanoparticles and thus reduces hemolysis and the suppressant effects of Bone marrow which leads to a blood-modulating effect on red blood cells (23).
5.1. Conclusions
The reported changes in white and red blood cells following the gavage of nanoparticles are possibly due to an immunogenic response (increased inflammation & ROS) and disturbances in signaling pathways and maturation of cells (dysfunction of the bone marrow, liver, and mitochondrial damage). These factors can have an effect on blood cells as well as the division and development of other cells. Also, silver nanoparticles increase inflammation and produce hepcidin which causes impaired absorption of iron and blood precursors and also they decrease the production of thrombopoietin which causes decrease in platelet count. It seems by reducing liver damage and inflammatory factors (free radicals) and thus reducing the production of hepcidin and increasing the production of thrombopoietin, the extract of S. striata modulates the levels of blood parameters (blood cells and platelets) due to the flavonoid compounds. Nevertheless, more research is needed to figure out the cellular and molecular processes that make S. striata extract protective.