1. Background
Back pain is a serious problem for all ages, especially the elderly. Years of life disability (DALY) due to back pain and neck pain had an increase rate of 59% from 1990 to 2015 (1) which relates to excessive stress and incorrect living habits (2-4). Asthe spine is a complex system consisting of vertebrae and cartilage that protects the spinal cord, remedies of back pain is expensive and difficult (4). In cases where non-invasive methods have not been successful, surgical methods such as disc replacement and vertebral ligation are recommended (5-8). Over the last 5 decades, orthopedic metal equipment has been used to correct the shape of the spine in scoliosis (6, 9-12). Orthopedic implants are mainly made of metal alloys that have shown good mechanical properties in the mentioned area (11). Stainless steel has also been used to repair the spine for a long time (10, 12). Recently, nitinol has attracted more attention due to its unique properties (13, 14).
Despite the good strength of metals, their young’s modulus is higher than that of bone, which in turn causes the stress shielding phenomenon, which is problematic. The Young's modulus of natural bone is about 0.5 - 20, nitinol 30 - 50, stainless steel 200 and cobalt-chromium-molybdenum alloy 240 GPa (15-17). In a study, nitinol was compared with Co-Cr and Ti alloy, in which Nitinol showes 100 times better wear resistance than Ti alloy (18). On the other hand, due to its special properties, nitinol has various applications in both industry and medicine. Including: Angiography, angioplasty, orthopedics, orthodontics, urology, guide wire and neurosurgery (19, 20). Noting the properties of NiTi, including its shape memory property due to the transformation of the martensite phase, and its super elasticity and biocompatibility properties, which made this alloy a suitable candidate for medical application (19). In another study in 2016 (20), the properties of thermo elastic and pseudo elasticity and shape memory of nitinol were stated. As mentioned, studies have shown that nitinol is one of the best choices for implants, especially in the neck and lumbar region (21). However, no immune stimulation was seen after nitinol placement. Therefore, it is a safe choice for implants (13).
So far, no research has been done on the use of nitinol alloy as a lumbar vertebral implant according to the patient's anatomy with the characteristics considered by the researchers of this project, so this study is completely original. Also, the techniques of making through sintering, while being simple and cost-effective, are completely new for producing such a piece.
Studies comparing porous nitinol and solid nitinol showed that porous nitinol has better bone formation, osteocunductivity and bioactivity than mass nitinol (22). So in this study we also used porosity. In the construction of orthopedic implants, the point that should be considered is the phenomenon of tension shield. Because orthopedic implants are generally made of metals and their alloys, metals also usually have a high modulus of elasticity and when placed in the body may lead to the phenomenon of tension shields. Therefore, to prevent this problem, the alloy modulus should be reduced, which is possible by creating porosity.
2. Objectives
In this study, to reduce the modulus of elasticity, the part must be porous, which can be done with a 3D printer and the addition of porous materials with high accuracy. And we hope to be able to design and provide a metal implant for the lumbar vertebrae that provides some of the special properties (including shock absorption, good mechanical properties) in the vertebral region that have been part of orthopedic medical engineering vacuums. This study will help orthopedic medicine.
3. Methods
In this study, after preparing nickel and titanium hydride powders with a purity of 99.5% and 99.9% and a particle size of 10 microns and less than 50 microns, respectively, purchased from Merck Germany, the powders were combined with urea to create porosity. 50% was mixed and then pressed with a press of 150 MPa, then the furnace was heated with argon atmosphere at 1050°C for 2 hours to sintering. It is worth mentioning that the exit of the atmosphere was done at a temperature of 200 degrees and 1 hour and also the decomposition of titanium hydride was done at a temperature of 500 degrees for 1 hour. Six sample with a diameter of 5 mm and a height of 7 mm was then obtained (Figure 1).
Also, for better patterning and tangibility of the implant, a plastic lumber vertebra was made by 3D printer method (Figure 2). Figure 2 was merely a mock-up made to better understand and feel the lumbar vertebrae.
The microstructure of the samples was examined by XRD. Since the sample should be biocompatible after placement in the body and should not cause an immune system reaction and degrade to adjacent tissue, this case was also examined and the samples were tested in vitro with an MTT kit and the biocompatibility was assessed.
3.1. Description of Experiments
Samples of lumbar vertebral implants made by sintering method were subjected to the following tests:
3.1.1. Investigation of Alloy Microstructure
XRD analysis (Seisert PTS3003) was used to ensure the alloying process and to study the phasic structure of the alloy. Under the situation of CuKα radiation and λ = 1.54. Scanning speed of 0.05 degrees per minute at an angular distance of 5 to 100 was selected.
3.1.2. Biocompatibility Review
In the next part, the sample was placed in contact with bone cells and the survival rate of the cells was evaluated and presented with MTT kit. The method is as follows: First, cells prepared in bulk in RPMI-1640 medium containing 50 units of penicillin and 50 micrograms of streptomycin per ml of culture medium supplemented with 10% of fetal calf serum in a culture flask in an incubator at 37°C and 5% carbon dioxide were cultured with 85% humidity. After 3 to 4 days (cell layer formation), the cells are removed from the surface of the flask by trypsin (0.25%) and a suspension with a concentration of 4 × 104 cells per ml is prepared for use. On the other hand, the sample was sterilized by an oven (dry heat). The samples were placed in a container of 12 cells (each sample separately in a cell) and one cell without a sample is considered as a control. After placing the samples in each house, 2 mL of cell suspension is poured and placed in an incubator. The MTT solution is then added to the wells and incubated again. (For the preparation of MTT solution at a concentration of 5 mg/mL, the amount of 50 mg of MTT powder is dissolved in 10 mg of 0.15 PBS molar. After 3 to 5 hours of incubation at 5°C, the cell supernatant is removed and instead 1 μL of isopropanol solution (Merck, Germany) is added to the relevant cavities. In this way, the corresponding plate chambers are placed on the shaker for 1 to 4 minutes. Their contents are then read by a microtiter in 2 nm. As seen, nitinol was biocompatible and good cell growth was seen from the sample.
4. Results
4.1. Result of Alloy Microstructure
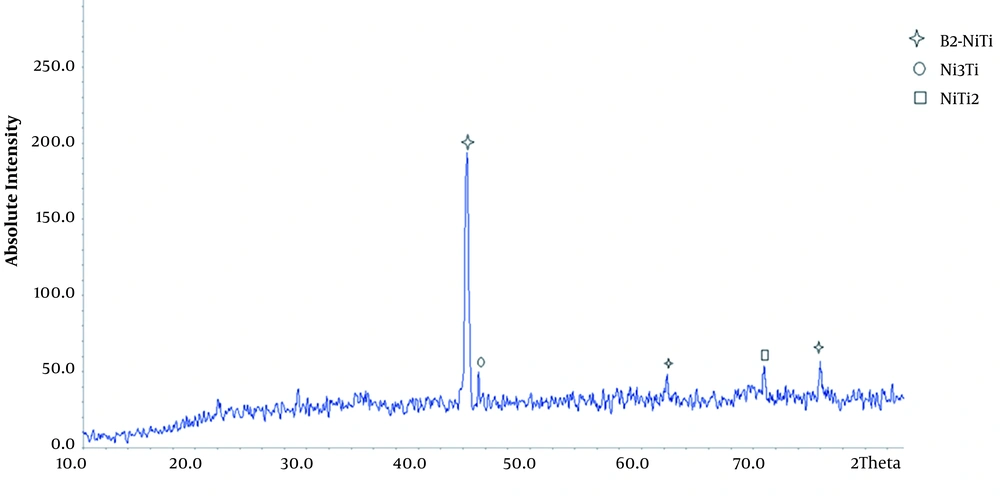
XRD images of porous nitinol samples are given. As can be seen in Figure 3, the predominant phase of the porous sample is B2-NiTi, and Ni3Ti and NiTi2 sediments are also present in the field.
The following reactions occur during the sintering process:
1. Ni + TiH2 → NiTi + H2 + Q
2. Ni + TiH2 → NiTi2 + H2 + Q
3. Ni + TiH2 → Ni3Ti + H2 + Q
According to the Ni-Ti phase diagram, Ni3Ti and NiTi2 sediments are stable compounds in the Ni-Ti binary system. The second and third tier reactions are thermodynamically more stable. As a result, it is difficult to prevent the formation of these sediments in the excavation process and they are usually seen in the structure of the excavated parts. The sample is also porous and porosity reduces the penetration of nickel and titanium. This increases the titanium and nickel-rich areas and the formation of sediments in these areas. According to the X-ray diffraction pattern, the sample has an austenitic structure. Increasing the percentage of nickel reduces the start temperature of the martensite phase (16, 23). Therefore, at ambient temperature, the samples have an austenitic structure.
4.2. Review of Biocompatibility Results
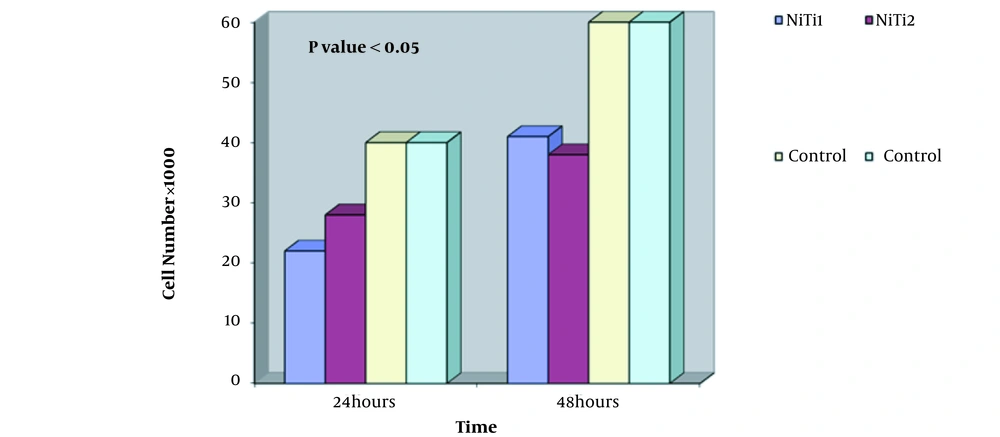
MTT test was performed on two samples (Figure 4). The graph shows the number of cells in terms of time of one day and two days. By examining the diagram and comparing cell viability on nitinol samples and controls, it can be concluded that the nitinol sample is slightly toxic and cell viability is good and appropriate, and has not caused cell death.
5. Discussion
According to the X-ray diffraction pattern, the sample has an austenitic structure. This is due to the oxidation of the titanium on the surface and the enrichment of the samples with nickel. Increasing the percentage of nickel reduces the starting temperature of the martensite phase, so at ambient temperature the samples have an austenitic structure. Above the austenite finishing temperature is the cubic nitinol structure B2. Below this temperature are rhombohedral, tetragonal, orthorhombic and monoclinic (24-27). Meanwhile, Ni-rich NiTi has unique memory properties due to BCC/rhombohedral structural changes (16, 28).
MTT test and cytotoxicity test showed good cell adhesion, which has various causes and many variables are involved (29). These properties have made this alloy superior to other alloys in orthopedic implant applications, especially in areas under continuous loading.
5.1. Conclusions
In this study, according to the experiments, it can be concluded that NiTi alloy can be suitable for being used as a material in implant manufacturing specially in spinal region. It is hoped that in the future we will work on different implant designs for orthopedic applications.