1. Background
Misuse and overuse of antibiotics or antimicrobial drugs result in the progression of antimicrobial resistance in the human population and have become one of the top 10 healthcare-related threats to the public across the world (1). Beta-lactamase-producing microorganisms are causative agents of nosocomial infections and also contribute to increased healthcare and economic burden due to prolonged hospital stays, increased mortalities, and hence increasing healthcare costs (2). World Health Organisation (WHO) has also listed antibiotic-resistant bacteria from 12 families as a high risk to public health (3). Bacillus is a globally distributed gram-positive, aerobic bacterium which forms spores and has a rod-shaped appearance (4). Due to endospore formation under unfavourable circumstances, Bacillus bacteria are found in clays, food, stones, sand, aquatic environments, soil, plants, and the gastrointestinal systems of countless animals and insects (5). Some of the most prevalent Bacillus species, including Bacillus subtilis and Bacillus licheniformis, are classified by US FDA and are commonly considered as safe (6).
Bacillus subtilis strains, model species for gram-positive organisms, can synthesize 2 dozen antibiotics with various structures and activities, based on the ecological niche and acquired systematic resistance (7). It has been discovered that several bacteriocins, lipopeptides, and other inhibitory substances having bacteriocin properties can be identified in Bacillus spp. (8). To protect themselves and the development of the same bacterial species, bacteria produce and release small antimicrobial peptides called bacteriocins. Bacteriocins mainly prevent bacterial development by creating pores on the surface of the cells or obstructing the production of cell walls (8, 9).
Bacteriocins of both class I and class II are produced by B. subtilis (10). Antibiotics in class I can undergo numerous posttranslational modifications, while ribosomally produced peptides in class II are smaller, pH- and heat-stable. Bacteriocins generated by Bacillus subgroups, including as ericin S, ericin A, and subtilin, have been demonstrated to only hinder the growth of gram-positive bacteria (11). It is thought that 99 % of bacteria and archaea can make at least 1 bacteriocin. Lactic acid bacteria (LAB) have long been explored as important bacteriocin makers, owing to their long history of safe use in food fermentation (4). Bacteriocins made from bacteria are utilized in the food industry, but researchers are looking into their potential as an infection-fighting antibacterial agent (12, 13). Bacteriocins have been researched because of their potential activities against multi-drug resistant bacteria, viruses, as well as some fungi too (14-16).
Bacteriocins can be employed as broad-spectrum antibiofilm medications with the potential for healing. They are typically secure and safe. Antimicrobial substances produced by several Bacillus strains have the potential to treat multidrug resistance issues (17-19).
2. Objectives
This study will investigate the inhibitory effect of B. subtilis bacteriocin on pathogenic bacteria.
3. Methods
3.1. Ethical
Ethical approval for this study was taken as IR.UMA.REC.1400.059.
3.2. Bacterial Isolation from Soil
For sampling, 5 soil samples were used and sent to the laboratory in plastic containers. In order to remove spore-free bacteria and germ cells, the collected samples were treated in a hot water bath for 10 minutes. Dilution was performed in different concentrations by using a physiological serum. The most recent agar was inoculated into nutrition culture media at various dilutions, and then the plates were incubated for 24 hours at 37°C. Colonies grown on an agar plate are studied for colony morphology, cell morphology, hot reaction and endospore formation (20).
3.3. Biochemical Test
Biochemical tests including; catalase activity, motility, glucose uptake by butanediol fermentation pathway (MRVP), citrate utilization and starch hydrolysis, Indole and oxidase tests, were performed according to Bergey’s manual of systematic bacteriology (21).
3.4. Molecular Identification
For the molecular identification of Bacillus strains that were biochemically confirmed, the 16s rRNA sequencing method was used where polymerase chain reaction was used to clone the 16s rRNA gene. Specific primer sequences were used. The first step was DNA preparation by manual method. Isolates of the bacteria were labelled. After that colonies were boiled for 15 minutes and centrifuged at high speed for 10 minutes at 4°C. A standard procedure was used to extract total DNA from the pellet. The PCR test was carried out using the prescribed primers following DNA extraction. The final PCR products were sent to the relevant companies for sequencing, and the submitted sequences were reviewed and finalized by comparison with the NCBI Genome Bank (22). The 16s RNA primer sequences used for PCR are:
Forward: 5’–AGAGTTTGATCCTGGCTCAG– 3’
Reverse: 5’– AAGGAGGTGATCCAGCCGCA– 3’
3.5. Preparation of Pathogen Samples
Standard samples of pathogenic bacetria; Streptococcus pyogenes, Salmonella typhi, Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa were obtained from a microbial bank either from available hospitals or samples in a research group.
3.6. Phenotypic Test for Microorganisms That Produce Beta-lactamases
3.6.1. MBL Identification by Phenotypic Confirmatory Testing
An EDTA solution with a concentration of 0.5 M was prepared by adding 46.53 g of disodium EDTA.2H2O into 250 mL of distilled water and adjusting the pH to 8.0 with NaOH (23). Autoclaving was used to disinfect the mixture. Ten grams of imipenem discs were placed on MH agar and one of them acquire 4 μL of an EDTA solution in order to achieve the necessary concentration. The zones of inhibition of the imipenem and imipenem-EDTA discs were evaluated after incubation of 16 to 18 hours at 35°C. The imipenem-EDTA disc in the combination disk test (CDT) had a 7 mm greater inhibitory zone than the imipenem disc alone, confirming the presence of MBL (24).
3.6.2. Assay for Phenotypic Confirmation of Extended-spectrum-lactamase Production
For phenotypic confirmation ceftazidime-clavulanic acid (30/10 mg), ceftazidime (30 mg), cefotaxime-clavulanic acid (30/10 mg) and cefotaxime (30 mg) were used in the CDT. As suggested by CLSI, isolates were tested using Mueller-Hinton agar and antibiotic-specific discs (Padtan Teb Co. Iran) (25). The inhibition zone diameter was confirmed to be phenotypically for extended-spectrum-lactamase (ESBL) production if it was 5 mm greater for clavulanic acid than without (26).
3.6.3. Antimicrobial Metabolite Extraction
The strains were cultured in liquid LB medium and then the Erlenmeyer flakes were placed in a 30°C oven for 24 hours. The whole culture medium was centrifuged at 6000 rpm and the supernatant was filtered through a filter. The final solution was mixed with ethyl acetate. After separating the solvent phase from the aqueous phase, the excess ethyl acetate was evaporated in a bath at 60°C. The extracted crude was stored at 4°C to investigate its antimicrobial activity (27, 28).
3.6.4. Investigation of Antimicrobial Activity of Standard Antibiotics
Antibiotic resistance of pathogenic strains was tested against some standard antibiotics, by using the disc-diffusion technique. Antibiotics used in the study include; ciprofloxacin, clindamycin, cephalexin and amoxicillin/clavulanate.
3.6.5. Investigation of Antimicrobial Activity of Extracted Crude
Disk diffusion was used to examine the crude extract of the strains’ antibacterial activity. First, a dense culture of the studied pathogens was prepared in Müller-Hinton agar medium, and sterile paper disks with a diameter of 6 mm and impregnated with 30 μL of the extracted crude extract were placed separately on the agar surface. As a negative control, a disc was soaked in 30 μL of ethyl acetate. The media were incubated for 24 hours at 37°C, and the diameter of zones of inhibition were evaluated to determine the antibacterial activity of the crude extracts (mm) (29).
3.6.6. Effect of Different Temperatures, pH and Incubation Times on Bacteriocin Activity
The stability and antimicrobial activity of bacteriocin were tested at different temperatures (25°C, 37°C, 45°C, 55°C and 65°C), different pH (2, 4, 7, 11, 14) and with different incubation times (12, 24, 36, 48, 60 and 72 h). The activity of extracted bacteriocin from B. subtilis was tested against each pathogenic bacteria (30).
4. Results
The diagnosis of B. subtilis was made using biochemical characterisation as shown in Table 1 which was further confirmed by 16s rRNA sequencing. The bacteriocin obtained from B. subtilis strains by ethyl acetate extraction method showed significant inhibitory effects on pathogenic bacteria. Bacteriocin activity against pathogens at different incubation times, temperatures, and pH were shown in Tables 2 - 4, respectively. Results for inhibition zones created by standard antibiotics and bacteriocin extracted from B. subtilis are presented in Tables 5 and 6, respectively. Table 6 results showed that Gram-positive bacteria were almost completely inhibited, and S. pyogenes was significantly inhibited by bacteriocin. Gram-negative bacteria were inhibited less than gram-positive bacteria, indicating that A. baumannii and K. pneumoniae were less inhibited against bacteriocin. Comparisons were made between bacteriocin activity and that of the common antibiotics ciprofloxacin, clindamycin, cephalexin, and amoxicillin clavulanate. When compared to the standard antibiotics, the crude extract of bacteriocin from B. subtilis strains significantly inhibited the growth of tested pathogens.
| Biochemical Characterisation | Bacillus subtilis |
|---|---|
| Motility | + |
| Catalase activity | + |
| Glucose uptake by MRVP | –/+ |
| Citrate utilization | + |
| Starch hydrolysis | + |
| Indole | – |
| Oxidase test | – |
| Incubation Time | Zone of Inhibition | ||||
|---|---|---|---|---|---|
| Acinetobacter baumannii | Pseudomonas aeruginosa | Streptococcus pyogenes | Salmonella typhi | Klebsiella pneumoniae | |
| 12 | 7.10 | 8.14 | 8.20 | 8.10 | 8.40 |
| 24 | 10.85 | 11.90 | 16.94 | 12.50 | 11.45 |
| 36 | 8.24 | 10.20 | 13.40 | 10.80 | 10.20 |
| 48 | 7.10 | 7.95 | 8.40 | 8.20 | 8.70 |
| 60 | - | - | 7.20 | 6.95 | 7.10 |
| 72 | - | - | - | - | - |
| Temperature | Zone of Inhibition in Pathogens | ||||
|---|---|---|---|---|---|
| Klebsiella pneumoniae | Salmonella typhi | Streptococcus pyogenes | Pseudomonas aeruginosa | Acinetobacter baumannii | |
| 25 | 10.20 | 10.64 | 14.20 | 11.94 | 10.40 |
| 37 | 13.52 | 14.67 | 17.45 | 13.98 | 11.95 |
| 45 | 11.30 | 11.40 | 12.24 | 11.70 | 10.98 |
| 55 | 8.20 | 9.51 | 9.20 | 8.92 | 8.80 |
| 65 | – | – | – | – | – |
| pH | Zone of Inhibition | ||||
|---|---|---|---|---|---|
| Acinetobacter baumannii | Pseudomonas aeruginosa | Streptococcus pyogenes | Salmonella typhi | Klebsiella pneumoniae | |
| 2 | 7.16 | 7.24 | 8.54 | 9.34 | 8.26 |
| 4 | 8.21 | 9.71 | 10.12 | 10.90 | 8.90 |
| 7 | 11.20 | 14.41 | 17.25 | 14.74 | 13.92 |
| 11 | 8.55 | 8.84 | 13.94 | 10.14 | 9.60 |
| 14 | 7.20 | 7.10 | 9.20 | 7.50 | 7.30 |
| Species | Inhibition Zone | |||
|---|---|---|---|---|
| Amoxicillin/ Clavulanate | Clindamycin | Cephalexin | Ciprofloxacin | |
| Acinetobacter baumannii | 13.57 | 14.64 | 13.10 | 14.80 |
| Pseudomonas aeruginosa | 14.96 | 15.45 | 14.72 | 14.67 |
| Streptococcus pyogenes | 16.82 | 16.46 | 15.60 | 15.92 |
| Salmonella typhi | 15.20 | 14.90 | 14.70 | 14.20 |
| Klebsiella pneumoniae | 15.91 | 15.86 | 16.54 | 14.60 |
| Bacterial Strain | Inhibition Zone |
|---|---|
| Klebsiella pneumoniae | 11.45 |
| Salmonella typhi | 12.50 |
| Streptococcus pyogenes | 16.94 |
| Pseudomonas aeruginosa | 11.90 |
| Acinetobacter baumannii | 10.85 |
4.1. The Effect of Incubation Time on Bacillus subtilis Bacteriocin Antimicrobial Activity
Different time intervals (12, 24, 36, 48, 60 and 72) were given for the incubation of samples. The maximum activity was obtained in 24 hours from 12 to 72 hours. In 24 hours, the largest area of inhibition of B. subtilis bacteriocin against S. pyogenes (16.94 mm) was greatest followed by S. typhi and P. aeruginosa (12.50 and 11.90 mm, respectively) was observed. The lowest inhibition zone was detected against A. baumannii (10.85 mm) in 24 hours. With increasing time, the activity of bacteriocin of B. subtilis was decreased as shown in Table 2. No antibacterial activity was observed after 72 hours of incubation. Such activity data might be observed due to the protein nature of bacteriocin.
4.2. The Effect of Temperature on Bacillus subtilis Bacteriocin Antimicrobial Activity
Samples were treated with different temperatures (25, 37, 45, 55 and 65). Treating bacteriocin at different temperatures caused the increase in the activity at 25 to 37°C by treating for 30 min. Bacteriocin activity decreased after heat treatment at 45°C and was lost at 65°C. Maximum antimicrobial activity was observed from a temperature range of, 25 to 65°C. At 37°C, the largest area of inhibition of B. subtilis bacteriocin against S. pyogenes (17.45) is observed followed by S. typhi and K. pneumoniae (14.67 and 13.52), respectively. The lowest inhibition zone, was detected against A. baumannii (11.95). The data showed that as the temperature was increased, and bacteriocin activity decreased since no antimicrobial activity was observed after 65°C.
4.3. Effect of pH on the Antibacterial Activity of Bacillus subtilis’s Bacteriocin
Samples were treated with different pH (2, 4, 7, 11 and 14). At pH 7, the largest area of inhibition of B. subtilis bacteriocin against S. pyogenes (17.25), followed by S. typhi and P. aeruginosa (14.74 and 14.41). The lowest inhibition zone, was detected against A. baumannii (11.20). Bacteriocin B. subtilis had the highest activity at neutral pH which is 7 and significantly reduced activity was observed at acidic and basic pH which is 2, 4, 11 and 14.
Inhibitory activity against standard antibiotics such as; ciprofloxacin, clindamycin, cephalexin and amoxicillin/clavulanate were shown in Table 5 to compare them with bacteriocin activity. The bacteriocin B. subtilis showed significant inhibitory activity against the tested pathogens compared to standard antibiotics, as shown in Table 6.
The largest area of inhibition of B. subtilis bacteriocin, was observed against S. pyogenes (16.94 mm), followed by S. typhi and P. aeruginosa (12.50 and 11.90 mm, respectively). The lowest inhibition zone was detected against A. baumannii (10.85 mm), as shown in Table 6.
Standard antibiotics such as ciprofloxacin, clindamycin, cephalexin and amoxicillin/ clavulanate were compared with bacteriocin activity. The bacteriocin B. subtilis showed significant inhibitory activity against the tested pathogens compared to standard antibiotics as shown in Table 6. The largest area of inhibition of amoxicillin/clavulanate (16.82) showed great antimicrobial activity for S. pyogenes followed by clindamycin and ciprofloxacin (16.46 and 15.92) respectively.
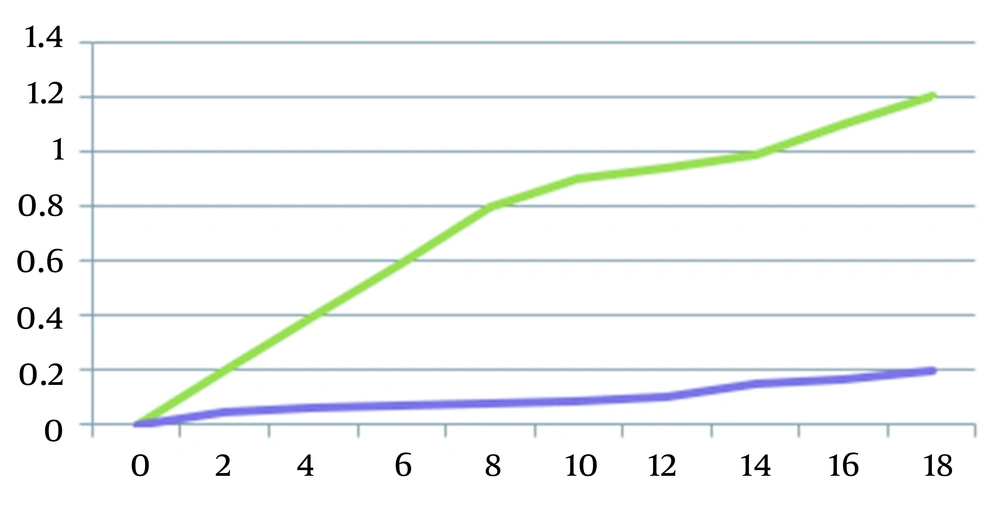
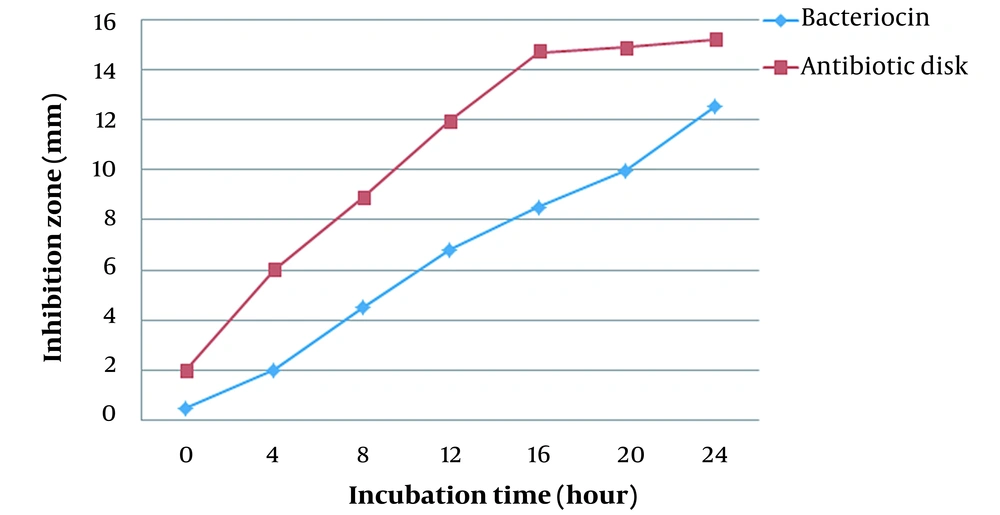
By plotting the bacterial growth curve, the effect of bacteriocin on S. pyogenes was investigated. To obtain growth curves for the bacteria, absorbance was measured at various intervals of time (0, 2, 4, 6, 8, 10, 12, 14, and 18 hours (Figure 1). Figure 2 shows comparing inhibition zones of S. typhi under the action of antimicrobial disk and bacteriocin.
5. Discussion
The bacteriocin from B. subtilis showed inhibitory effects on antimicrobial-resistant pathogenic microbes like; S. pyogenes, S. typhi, P. aeruginosa, K. pneumonia, and A. baumannii. Of which, bacteriocin showed the highest antibacterial activity against gram-positive pathogenic bacteria, S. pyogenes. Among gram-negative pathogens used in this study, bacteriocin showed high antibacterial activity against S. typhi, followed by a similar kind of inhibitory effect against P. aeruginosa and K. pneumonia. While the lowest antibacterial activity was observed against gram-negative bacteria A. baumannii. Numerous studies have identified bacteriocins from different microbial sources and studied their antibacterial activity against distinct bacterial species (31). Colicin was the first bacteriocin discovered, in 1925. Later researchers discovered that many gram-positive, as well as gram-negative bacteria have a widespread ability to create these antimicrobial peptides. These compounds are meant to provide a competitive advantage to their producers over other microbes (14, 32). Bacteriocins are a heterologous group of proteinaceous antibacterial compounds produced by bacteria of all main lineages and are synthesized by ribosome synthesis. They show differential antimicrobial potency, sizes, structures, immunity mechanisms, and modes of action (33, 34). They have a high level of target specificity towards closely related bacteria, even though many of them have a broader range of activity (35).
Bacillus subgroups have been found to produce a wide range of bacteriocins with different molecular weights as a result. Bacillus, soil-dwelling bacteria, was discovered to be capable of producing many antimicrobial chemicals that were also determined to be safe to use (36). The following points set bacteriocins apart from antimicrobial drugs: (1) bacteriocins are produced on the ribosomal surface of bacterial cells, whereas antibiotics are secondary metabolites of bacteria (37); (2) antibiotic producers are susceptible to antimicrobial agents, while bacteriocin producers are resistant to antimicrobial agents (38); (3) since the target bacterial cell surface lacks any specialized receptors, bacteriocins can attach to the bacterial cell surface everywhere (17).
There is a wide range of bacteriocins found in B. subtilis which are referred to as class I and II. Class I can undergo post-translational modifications and class II ones are small, ribosomally synthesised peptides, which show pH- and heat-stability (7). It’s vital to identify real bacteriocin ribosomal production in the instance of Bacillus, because this bacterium is notorious for producing antimicrobial peptides via non-ribosomal synthesis too. It is presently predicted that at least 4 - 5% of the genome of any B. subtilis strain is dedicated to the production of antimicrobial compounds (AMCs) (39).
The Bacillus genus sensu lato produced bacteriocins and BLIS, are most likely second in importance only to LAB-produced bacteriocins. Strains of the Bacillus genus produce a variety of antimicrobial peptides with various fundamental chemical structures (8, 40). As bacterial resistance to conventional antibiotics in clinical use increases, bacteriocins are being evaluated as a substitute for antibiotics used to treat human diseases (41). Cross-resistance between frequently used antibiotics and bacteriocins have been uncommon since these 2 forms of antibiotics focus on different biological targets. Bacteriocins, also known as BLIS, are produced by Bacillus species and exhibit antibacterial efficacy against harmful bacteria including VRE and MRSA. Examples include the lantibiotics, haloduracin, or the BLIS produced by Bacillus sphaericus (42).
In both human and veterinary medicine, the ESBL-producing Enterobacteriaceae has become an issue. A resistance mechanism in Enterobacteriaceae that lowers the effectiveness of expanded spectrum cephalosporins and monobactams is currently of attention (43, 44). Bacteriocins produced by lactic acid bacteria have come to light as potential substitutes for food preservatives as a result of this circumstance because they exhibit inhibitory activity against MDR pathogens (44). Although bacteriocins are typically quite strong, they only work against organisms that are phylogenetically related to the bacteria that produce them (45). When a strain that does not produce bacteriocin, contains a gene similar to the self-defence gene of bacteriocin-producing bacterium, mimicking natural defence immunity takes place. When bacteria are attacked, they release enzymes that break down bacteriocin peptides; a defensive molecule called nisinase, is produced by Bacillus cereus and Paenibacillus polymyxa, responsible for the breakdown of nisin (46).
More studies showed bacteriocins are produced by B. subtilis and have antibacterial effects against some human and animal pathogens, including multidrug-resistant ones too. A bacteriocin named Bacillion22, was isolated and purified from B. subtilis which showed antimicrobial activity against some food-borne pathogens (47). A recent study showed that marine B. subtilis (BacSM01) can significantly suppress the growth of methicillin-resistant Staphylococcus aureus as well as ESBL-producing gram-negative pathogens like; A. baumannii, P. aeruginosa, and Escherichia coli (30). In another study, B. subtilis isolated from soil samples showed antibacterial activity against 4 types of diabetic foot ulcer-causing pathogens; Pseudomonas spp., Staphylococcus spp., Klebsiella spp. and Proteus spp. The partially purified bacteriocin from B. subtilis showed high antibacterial activity against Klebsiella spp. (9). Plant-derived B. subtilis MK733983 strain showed antibacterial activity against a broad-range of bacteria; S. aureus, P. aeruginosa, K. pneumoniae, E. coli and Chromobacterium violaceum and highest antimicrobial activity was observed against Mycobacterium smegmatis (48). Bacteriocin isolated from soil; isolate B. subtilis GAS101, showed good inhibitory activity against both gram-positive and gram-negative indicator bacteria Staphylococcus epidermidis and E. coli. Bacteriocin showed a good broad-range of antimicrobial activity along with anti-biofilm activity (19). In one research it was revealed that B. subtilis KKU213 strain produce Subtilosin A; which is a mixture of extracellular antibacterial peptides, exhibited inhibitory activity against B. cereus, Listeria monocytogenes, Micrococcus luteus, and S. aureus (49).
Some studies have evaluated the effects of different incubation times, pH and temperatures and also the action of some chemical compounds (proteolytic and non-proteolytic) on bacteriocin activity against pathogens. Additionally, the duration of incubation is crucial for bacteriocin activity. Bac-maximal SM01’s antibacterial activity was reached in the BHIB medium after 24 hours. Bac-SM01 generated either lost its activity during incubation or became unstable at 72 hours as evidenced by the inability to detect the antibacterial activity of the bacteriocin at that time (30). Bacteriocin from soil isolate B. subtilis GAS101 showed pH and temperature stability in its activity at temperature ranges of 30 - 121°C and pH ranges of 2 - 12 (19). Similarly, bacteriocin isolated from B. subtilis soil isolate which showed phylogenetic similarity with B. subtilis BSF01 showed stability in its activity at a temperature range of 40 - 100 °C and even at both acidic and basic pH with a high level of activity at acidic pH (9). While in another study, bacteriocin isolated from B. subtilis strain RLID 12.1, maximum activity was observed at pH range 6.0 - 8.0, while the stable and maximum activity was noted at 37°C and 80 - 90% of activity at temperature range 50 - 100 °C (50). Another research too showed differential activity of bacteriocin isolated from B. subtilis at incubation time, pH and temperature of, 24 h, 7, and 37°C, respectively (30). This differential stability of bacteriocin activity showed a similar type of variability in the results obtained in this study.
In this study, bacteriocin showed a wide variety of activity when exposed to different temperatures, pH levels, incubation times, and antibiotics. For differential parameters of incubation time, pH and temperature, maximum bacteriocin inhibitory activity was observed at an incubation time of; 24 h, pH 7.0, and a temperature of 37°C. While considerably good bacteriocin activity was observed, between 24 - 37 hours of incubation time, 4 - 11 pH ranges, and 25 - 45 temperature ranges. Standard antibiotics ciprofloxacin, clindamycin, cephalexin, and amoxicillin-clavulanate were used and compared with an inhibitory effect of bacteriocin against selected pathogenic strains. When compared to standard antibiotics, the bacteriocin B. subtilis demonstrated a significant inhibitory effect against the pathogens studied especially, at 24 hours of incubation time, at neutral pH of 7.0 and 37°C of temperature. The largest area of inhibition of B. subtilis bacteriocin was against S. pyogenes. The lowest inhibition zone, was detected against A. baumannii.
The use of bacteriocins as a medicinal agent against human diseases is still in the research and development stage, but they are already being used commercially for food preservation and as a probiotic. This is causing great enthusiasm in the scientific and medical communities. Bacteriocins can be used in conjunction with antibiotics to lessen undesirable side effects while maintaining antibiotic efficiency. Additionally, this would aid in halting the emergence of bacteria resistant to bacteriocin and antibiotics (51). According to WHO and World Bank reports, antimicrobial resistance poses a serious threat to public health, which might increase further by approximately estimated deaths of 10 million people, by 2050, making it a burden on the healthcare system and economy of the countries (52). Hence, there is a dire need for alternative medicines and bacteriocin produced by B. subtilis can be considered a better alternative to traditional antibiotics to treat antibiotic-resistant pathogen-related infections.
5.1. Conclusions
The current study’s findings lead us to conclude that bacteriocin isolated from B. subtilis obtained from soil samples can be a significant chemical compound for bacterial pathogen control. As an alternative to standard antibiotics, this molecule is very specific. Due to different types of bacteriocins produced by B. subtilis isolated from various sources, the differential activity and stability are observed at varying parameters like; temperature, pH and incubation time. Hence, further investigation is essential to study the chemical nature or class (I or II) of bacteriocin produced by B. subtilis in this study. Considering that, the bacteriocins are produced as a competitive strategy, which acts against closely related bacteria. This might be a reason the good inhibitory activity of bacteriocin was observed against gram-positive bacteria than gram-negative ones. Further analysis of, the chemical nature of extracted bacteriocin can help to shed a more light on it.