1. Background
The outbreak of Covid-19 began in Wuhan City, China on 31 December 2019 and has since become a global pandemic. Among the various complications associated with COVID-19 disease, decreased arterial blood oxygen saturation is considered one of the most dangerous. Arterial blood oxygen saturation reflects the amount of oxygen molecules bound to hemoglobin in red blood cells, which enter the bloodstream through the lungs during respiration. If the coronavirus affects the lungs, it can lead to a reduction in the amount of oxygen a person receives (1). Insufficient oxygen supply to the body can result in severe damage to vital organs such as the brain and liver within a few minutes (at most 3 minutes) after the onset of symptoms. Hence, monitoring oxygenation is crucial in COVID-19 patients.
There are two common methods to measure blood oxygen saturation: Arterial blood gas analysis (ABG) and pulse oximetry (2, 3). ABG measures the lung's efficiency in delivering oxygen to the bloodstream and removing carbon dioxide (Sao2). This test is usually conducted by sampling arterial blood from the wrist (4, 5). On the other hand, pulse oximetry is a non-invasive method that estimates oxygen saturation in the blood (Spo2). In this procedure, a device emits red light through the finger (or sometimes the earlobe or foot), and the amount of light absorbed by pulsating blood is measured. This measurement represents the percentage of hemoglobin cells saturated with oxygen (6). Several studies have examined the agreement between SpO2 and SaO2 (4, 7, 8), showing a strong correlation with a bias ranging from -0.70 to +1.86% (8).
Since the onset of the COVID-19 pandemic, concerns have been raised regarding the agreement between SpO2 and SaO2 in COVID-19 patients. Wilson-Baig et al. conducted a retrospective, single-center study involving 17 intensive care unit patients, which demonstrated an average underestimation of SaO2 by SpO2 of 5.3% (9). These findings raised the hypothesis that a COVID-19 infection might influence the agreement between SaO2 and SpO2. However, the lack of a control group in the Wilson-Baig study limited the significance of their results.
2. Objectives
Considering that arterial blood oxygen saturation is a critical parameter in managing acute respiratory failure globally, particularly during crises and in developing countries, and given the limited research on this vital issue, we conducted a study to assess the concordance of arterial oxygen saturation (SaO2) and pulse oximetry (SpO2) in both COVID-19 and non-COVID-19 patients admitted to the intensive care unit (ICU).
3. Methods
3.1. Type of Study, Study Population and Study Implementation Method
In this cross-sectional descriptive-analytical study, all patients with and without COVID-19 hospitalized in the ICUs of medical-educational centers in Imam Khomeini and Golestan hospitals in Kermanshah city, were investigated with the "convenience sampling" sampling method from September 19th, 2020 (9/19/2020) to March 18th, 2021 (03/18/2021). Blood samples were taken routinely whenever arterial blood gas analysis was required after a written consent was obtained from patients, and pulse oximetry was simultaneously recorded. A total volume of 2 ml of heparinized blood was first taken during routine arterial blood sampling. The arterial blood gas analysis of blood samples was then performed. The blood oxygen saturation level was simultaneously recorded using a pulse oximeter. The pulse oximeter probe was attached to the patient's finger with the best wave. Patients' oxygenation index was calculated in all patients (during hospitalization) with and without COVID-19 using two variables, i.e., SpO2 gained from pulse oximetry and SaO2 derived from arterial blood gas analysis. The degree of overlap between the obtained values in the two groups with the patient's clinical condition was examined.
The inclusion criteria for the study included definite diagnosis of COVID-19 (for the group of patients with COVID-19) with the result of polymerase chain reaction (PCR) or CT scan, hospitalization in the intensive care unit, need for mechanical ventilation, age 15 years and older.
Patients excluded from the study:
• Patients with acute coronavirus disease
• Patients with heart failure whose SpO2 level cannot be properly assessed by pulse oximetry due to hypotension
• Patients receiving methylene blue
• Patients with blue and black nail polish
• Patients with methemoglobinemia
• Patients with cold extremities and severe shivering
• Pregnant women
3.2. Data Analysis
The obtained data were analyzed using SPSS. 23.00 software. The quantitative and qualitative data were respectively reported as mean (SD) and frequency (percentage) for each group of the studied patients. The Kolmogorov-Smirnov normality test was used to examine if data are normally distributed in each group. Independent t-test was used to compare the variables in the two groups. Moreover, the Pearson correlation coefficient was used to measure the association between the given variables means in each group. In this study, the significance level was considered P < 0.05.
4. Results
In the present study, 60 patients with COVID-19 and 57 patients without COVID-19 hospitalized in the ICU were studied. The mean (SD) for disease incidence in COVID-19 infected patients hospitalized in the ICU was 7.12 (2.06) days. The mean (SD) for the number of hospitalization days in COVID-19 infected patients and other patients hospitalized in the ICU was 4.57 (2.22) and 3.12 (4.1), respectively. A total of 35 (58.3 %) patients with COVID-19 and 21 (36.8 %) patients without COVID-19 hospitalized in the ICU had a history of at least one type of previous diseases of which HTM (30%) and chronic obstructive pulmonary disease (COPD) (11.7%) was the highest frequent one among COVID-19 infected patients, respectively. The highest percentage of COVID-19 incidence was among male (60%) married patients (76 %). A total of 11 (18%) patients had a history of addition and 9 (15%) patients were cigarette smokers (see Table 1). Overall, 35 (58.3%) patients with COVID-19 and 15 (26.3%) patients without COVID-19 had a history of taking at least one type of medication (see Table 1). Table 2 shows the frequency, percentage, and history of the type of taken medication by the studied patients (with and without COVID-19). The most frequent type of medication used in patients with and without COVID-19 was lozartan (Table 2). Table 3 shows the type of clinical symptoms and their frequency. The predominant clinical symptoms in COVID-19 infected patients were shortness of breath (50%), cough, and weakness (48% for each one), respectively (Table 3). The mean and standard deviation of the clinical characteristics of patients are shown in Table 4. According to the results the mean (SD) value of oxygen saturation gained from pulse oximetry in patients with and without COVID-19 was 85.89 (13.34) and 96.02 (2.73), respectively. Moreover, the level of oxygen saturation derived from arterial blood gasses in patients with and without COVID-19 was 83.94 (16.12) and 94.96 (3.13), respectively. According to the independent t-test, there was a statistically significant difference in the means of oxygen saturation gained from pulse oximetry (MD = -10.13 (-6.52 to – 13.74)) and oxygen saturation derived from arterial blood gasses (MD = -11.02 (-6.78 to -15.25)) between the two groups of the studied patients (Table 4). Table 5 displays the correlation coefficients between the mean oxygen saturation gained from pulse oximetry (Spo2) and the mean oxygen saturation derived from arterial blood gasses (Sao2) by two groups of patients. As can be observed, there was a significant and relatively strong positive correlation between the mean oxygen saturation gained from pulse oximetry (Spo2) and the mean oxygen saturation derived from arterial blood gasses (Sao2) in COVID-19 infected patients (P < 0.05, r = 0.727). Moreover, a significant and relatively weak positive correlation (less than 5) was observed between the mean oxygen saturation gained from pulse oximetry (Spo2) and the mean oxygen saturation derived from arterial blood gasses (Sao2) in patients without COVID-19 (P < 0.05, r = 0.459) (Table 5).
| Variables | Patients with COVID-19 (n = 60) | Other Patients (n = 57) |
|---|---|---|
| Age (y) | 65.93 ± 14.54 | 43.37 ± 19.59 |
| Weight (kg) | 70.47 ± 9.29 | 71.47 ± 10.99 |
| Gender | ||
| Female | 24 (40.0) | 20 (35.1) |
| Male | 36 (60.0) | 37 (64.9) |
| Marital status | ||
| Single | 0 (0.0) | 17 (29.8) |
| Married | 46 (76.7) | 37 (64.9) |
| Divorced or deceased spouse | 14 (23.3) | 3 (5.3) |
| Previous medical history | ||
| No disease | 25 (41.7) | 36 (63.2) |
| Asthma | 2 (3.3) | 0 (0.0) |
| COPD | 7 (11.7) | 4 (7.0) |
| Diabetes | 4 (6.7) | 8 (14.0) |
| HTM | 18 (30.0) | 6 (10.5) |
| IHD | 2 (3.3) | 0 (0.0) |
| CVA | 2 (3.3) | 0 (0.0) |
| HIV | 0 (0.0) | 2 (3.3) |
| Tumor | 0 (0.0) | 1 (1.8) |
| History of medicine use | 35 (58.3) | 16 (28.1) |
| History of addiction | 11 (18.3) | 18 (31.6) |
| History of smoking | 9 (15.0) | 9 (15.8) |
aValues are expressed as Mean ± SD or No. (%).
| Type of Drug Used | Patients with COVID-19 ( n = 60) | Other Patients (n = 57) |
|---|---|---|
| Glibenclamide | 2 (3.3) | 0 (0.0) |
| Metformin | 4 (6.7) | 5 (8.9) |
| Atorvastatin | 2 (3.3) | 0 (0.0) |
| Losartan | 15 (25/0) | 6 (10.7) |
| Captopril | 2 (3.3) | 0 (0.0) |
| Salbotamol | 7 (11.7) | 1 (1.8) |
| Methoral | 3 (5.0) | 0 (0.0) |
| Serofolo | 1 (1.7) | 0 (0.0) |
| Prednizolon | 1 (1.7) | 1 (1.8) |
| Zede HIV | 0 (0.0) | 1 (1.8) |
| Ansolin | 0 (0.0) | 1 (1.8) |
| Clinical Symptoms | Patients with COVID-19 (n = 60) | Other Patients (n = 57) |
|---|---|---|
| Chest pain | 16 (26.7) | 1 (1.8) |
| Shortness of breath | 50 (83.3) | 10 (17.5) |
| Cough | 48 (80.0) | 12 (21.1) |
| Weakness | 48 (80.0) | 24 (42.1) |
| Pain | 21 (35.0) | 8 (14) |
| Headache | 13 (21.7) | 11 (19.3) |
| Tremors and sweating | 20 (33.3) | 2 (3.5) |
| Nausea and vomiting | 18 (30.0) | 5 (8.8) |
aValues are expressed as No. (%).
| Clinical Characteristics | Infected | un-infected | Mean Difference (95% CI) | P Value |
|---|---|---|---|---|
| Hb | 13.33 ± 8.72 | 13.03 ± 2.42 | 0.29 (-2.1 to 2.66) | 0.803 |
| pH | 7.33 ± 0.11 | 7.35 ± 0.07 | -0.02 (-0.05 to 0.02) | 0.363 |
| FBS | 153.64 ± 70.35 | 133.53 ± 74.02 | 20.11 (-6.36 to 46.58) | 0.135 |
| Patient temperature | 39.01 ± 7.79 | 36.73 ± 0.48 | 2.29 (0.27 to 4.30) | 0.029 |
| Heart rate | 93.45 ± 19.25 | 90.25 ± 14.45 | 3.20 (-3.01 to 9.42) | 0.309 |
| Systolic blood pressure | 130 ± 21.64 | 115.19 ± 17.94 | 14.81 (7.51 to 22.11) | < 0.001 |
| Diastolic blood pressure | 80.65 ± 13.76 | 73.57 ± 12.93 | 7.08 (2.16 to 11.98) | 0.005 |
| Spo2 | 85.89 ± 13.47 | 96.02 ± 2.73 | -10.13 (-13.74 to -6.52) | < 0.001 |
| Sao2 | 83.94 ± 16.12 | 94.96 ± 3.13 | -11.02 (-15.25 to -6.78) | < 0.001 |
aValues are expressed as Mean ± SD.
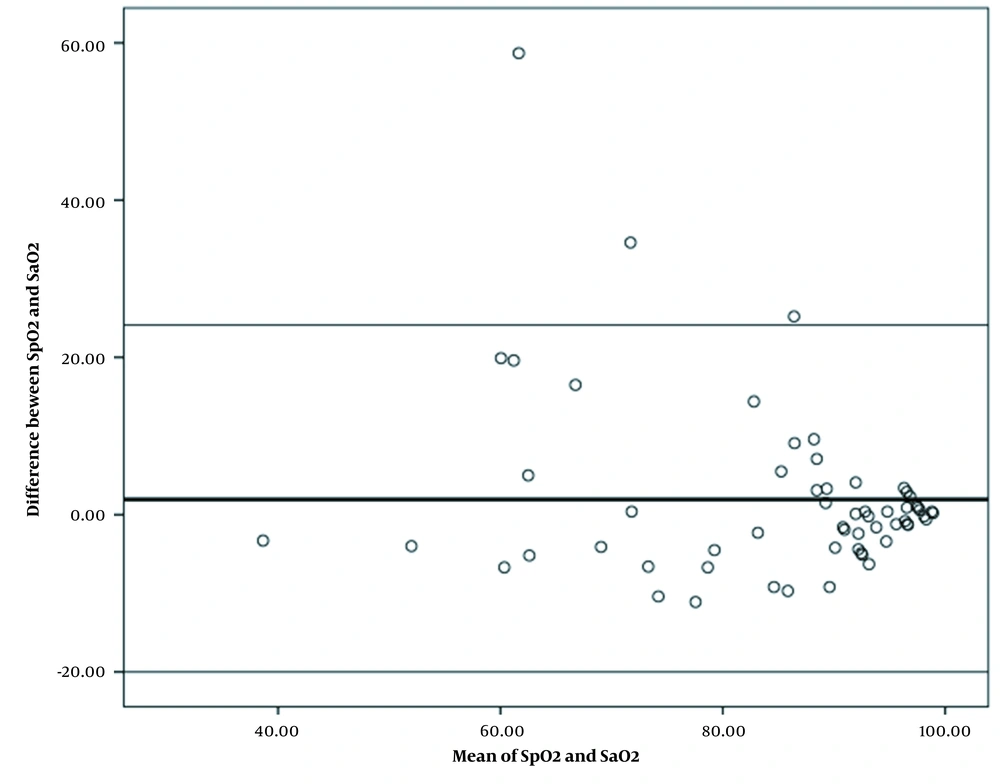
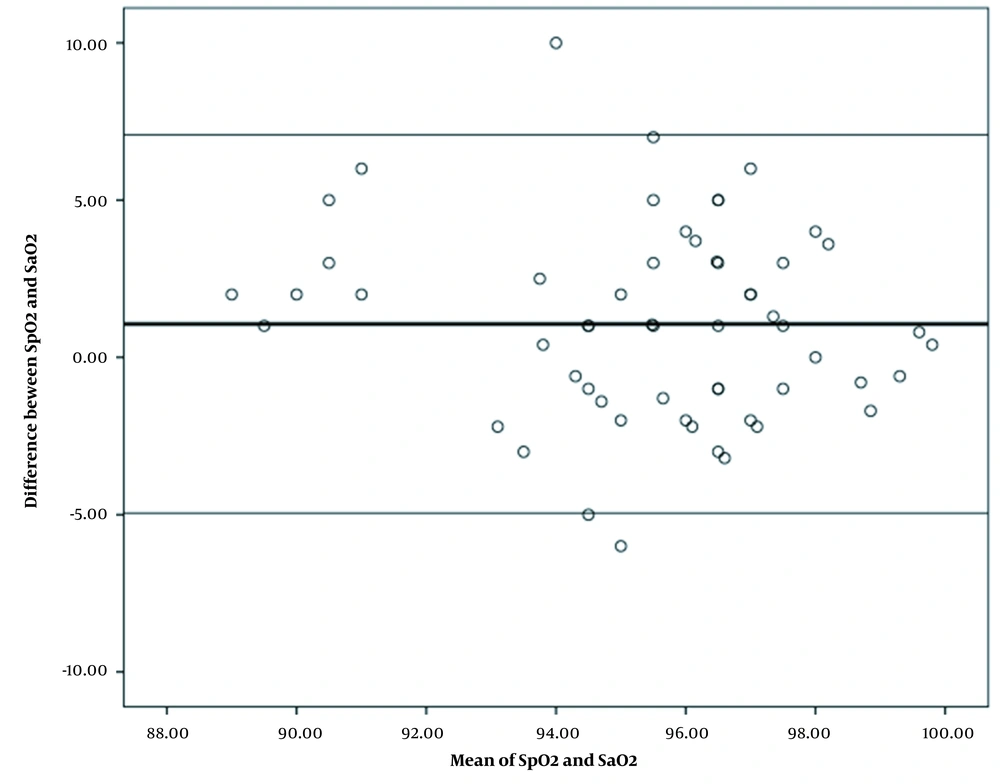
The mean (SD) value of oxygen saturation gained from pulse oximetry and oxygen saturation derived from arterial blood gasses was 85.89 (13.34) and 83.94 (16.12) in patients with COVID-19, respectively. The mean oxygen saturation gained from pulse oximetry with a difference of 1.95 was significantly higher than the mean oxygen saturation derived from arterial blood gasses. Bland-Altman statistical method also showed the mean difference (bias) of 1.95 with the confidence interval of 95% (-20.01 to 24.11) between the two methods of measuring the oxygen saturation in patients with COVID-19, and there was a relatively good agreement between oxygen saturation scores obtained using the two measurement methods in these patients. According to this figure, most of the points are distributed around the mean in the 95% confidence interval and the correlation between points of the two oxygen saturation measurement methods was higher at higher values of oxygen saturation percentage. Moreover, only three samples were not within the confidence interval (Figure 1). The mean (SD) value of oxygen saturation gained from pulse oximetry and oxygen saturation derived from arterial blood gasses in patients without COVID-19 was 96.03 (2.73) and 94.96 (3.12) respectively. The mean oxygen saturation gained from pulse oximetry with a difference of 1.06 was significantly higher than the mean oxygen saturation derived from arterial blood gasses. Bland-Altman statistical method showed the mean difference (bias) of 1.06 with the confidence interval of 95% (-4.95 to 7.08) between two oxygen saturation measurement methods in patients with COVID-19 and there was a relatively good agreement between oxygen saturation scores obtained using the two measurement methods in patients without COVID-19. The mean difference of measuring oxygen saturation percentage by pulse oximetry method with arterial blood gasses measuring method showed that the dispersion of data was low and most of the points were within the 95% confidence interval and only three samples were not within the given confidence interval (Figure 2).
5. Discussion
This study was conducted with the aim of assessing the concordance of arterial oxygen saturation (SaO2) and pulse oximetry (SpO2) in patients with and without COVID-19 hospitalized in the intensive care unit (ICU). The results of this study showed that there is a significant statistical difference between the blood oxygen saturations by pulse oximetry method and arterial blood gasses analysis (P < 0.05). Additionally, there was a significant and relatively strong positive correlation between the mean oxygen saturation Spo2 and Sao2 in COVID-19 infected patients. This correlation was significantly positive, but relatively weak, in normal patients. This indicates that due to the unstable oxygen level in patients with COVID-19 caused by the lungs involvement, pulse oximetry method can be used for rapid patient examination and initial clinical decision making. The patient's oxygen level can be measured more accurately with the help of the blood gas analysis. Altman statistical method also showed a bias of 1.95 with the confidence interval of 95% (-20.01 to 24.11) between two methods of measuring the oxygen saturation in patients with COVID-19. Accordingly, there is no significant difference in terms of the accuracy of pulse oximetry compared to the arterial blood gasses analysis in cases with high oxygen saturation levels in the COVID-19 infected patients. Therefore, SPO2 is not a true indication of SaO2 at low blood oxygen levels. In a study with a different approach, Mahoori et al. compared oxygen saturation measured by pulse oximetry and arterial oxygen saturation in patients admitted to the open heart ICU. They concluded that the mean difference between the pulse oximetry oxygen saturation and arterial oxygen saturation was 1.6 ± 0.12. Moreover, they observed a significant relationship between SaO2 and SpO2 in patients with normal hemoglobin levels. This relationship was also significant between patients with mild acidosis. The difference between SpO2 and SaO2 was 1.5 ± 0.05% that differed from the value obtained in the present research. The obtained data showed that in patients with stable hemodynamic and good pulse oximetry signal quality, pulse oximetry more reliably shows SaO2. Therefore, the pulse oximeter is a useful monitoring device for oxygen saturation in patients with stable hemodynamic (2). Inconsistent with the results from the present study, Wilson-Biag et al. concluded that oxygen saturation gained from pulse oximetry was lower than that derived from arterial blood gas analysis (9), while this value was higher than SaO2 for both groups of patients with and without COVID-19 in the present research. Several studies have shown that peripheral oxygen saturation can underestimate SaO2 in low perfusion states, arrhythmias, vasoconstriction, venous pulsations, edema, and severe anemia (10, 11). The nail polish can interfere with pulse oximetry signals and result in an inaccurate reading of oxygen saturation (11). The elevated blood levels of glycosylated hemoglobin (HbA1c) lead to an overestimation of SaO2 by SpO2 (12). In patients with sepsis and septic shock, there are conflicting reports on how SpO2 is biased (10, 11). The results obtained from another study indicated that SpO2 values correspond with SaO2 and are not affected by ethnicity (13). A study by Gurun Kaya et al. showed that pulse oximeters may not be suitable to evaluate the actual level of oxygen saturation, especially in COVID-19 infected patients with high levels of ferritin and fibrinogen and lower lymphocyte count with a low SpO2 reading (14). According to the findings from the present study, both groups of the studied patients experienced shortness of breath, cough, and weakness. Headache and chest pain in the COVID-19 infected patients and chest pain, trembling and sweating in normal patients had the lowest prevalence. Symptoms, such as pain, nausea, and vomiting were moderately prevalent in both groups. The results obtained from a similar study revealed that the risk of mortality has increased in the elderly because they are more prone to death and the underlying conditions are more prevalent among them. The most prevalent clinical symptoms upon the arrival were shortness of breath, cough, and fever. Blood oxygen saturation testing shows that most of these patients had SpO2 lower than 93%. The most prevalent symptoms in the deceased patients were shortness of breath and disturbance in the level of consciousness. Moreover, most patients needed oxygen therapy and were hospitalized in the ICU (15). In another study, Yang et al. stated that in Wuhan, China, the mean age of the patients who died from COVID-19 infection was 50 years, and most of the deceased patients were male. Approximately, 81% of patients showed mild symptoms and only 14% of them experienced severe symptoms, such as pneumonia and shortness of breath. About 5% of severe cases experienced respiratory failure and infectious shock and failure of other organs of the body. Consistent with the present study, fever and cough were reported as the most common symptoms, especially in children (16). While this study sheds light on important aspects of patient care, it is crucial to acknowledge its limitations. The restricted sample size and reliance on initial information impose constraints on the generalizability and accuracy of the findings. Moreover, the need to investigate the trends and changes in Spo2 and Sao2 levels throughout hospitalization emphasizes the significance of future studies in this area. Addressing these limitations will enhance our understanding of the subject matter and contribute to the improvement of patient care strategies.
5.1. Conclusions
Overall, the results from this research showed that there is a significant correlation in terms of blood oxygen saturation level based on SpO2 and SaO2 values between both groups of patients with and without COVID-19, but the difference is that pulse oximetry is not a proper method to measure the blood oxygen saturation level in patients with COVID-19 and this method can be acceptable in stable body conditions. Therefore, arterial blood gas analysis is suggested to be used to measure the actual level of oxygen saturation in patients with COVID-19. Although previous studies have obtained consistent and inconsistent results in this regard, it can be said in general that pulse oximetry is not a proper method to measure the blood oxygen level in patients with acute conditions.