1. Background
Breast cancer (BC) is one of the most common cancers and health problems in women under the age of 40, with a number of about 244 000 cases per year (1-4). In such a way that it constitutes more than 30% of important cancers in women (150 times more than men). The findings of the last few decades show that BC is the second cause of death in developing countries (44,800 deaths per year) and the third cause of death in developing countries (5-9). Breast cancer is caused by the abnormal proliferation of breast tissue cells, which mainly occurs in milk ducts and milk-producing glands, and may metastasize to distant areas of the body, or invade surrounding tissues (10). The world health organization (WHO) estimates that by 2050, at least 3.2 million women will be diagnosed with cancer (11, 12). The most frequent sites for BC metastasis include the bone, lung, liver, brain, and distant lymph nodes (13). The prevalence of BC in Iranian women is relatively high and accounts for 76% of common cancers in women (14). The researchers' findings show that the incidence of BC in the countries of Northern Europe and North America is higher than in Asia, Africa, South America and Southern Europe. This can be caused by the following factors: Aging, genetics, unhealthy behavior, types of radiation, smoking, geographical effects, number of pregnancies, late menopause, early menstruation, obesity, and differences in fertility patterns (15-19). Considering that various studies show that biological factors can be involved in 16% of human cancers (20). Women under the age of 45 account for 11 and 9% of BC in the United States and the United Kingdom (21). The cause of this increase can be due to various reasons such as changes in the status of social economic factors, increase in environmental risk factors, or improvement of diagnosis methods. Breast cancer can impose a lot of costs on the health system. Therefore, identifying the factors involved in the occurrence of cancer can be useful for the health system. Human papillomavirus (HPV) is a well-known sexually transmitted virus (22-25) that is primarily associated with cervical cancer, followed by anal, penile, and head and neck cancer. However, new evidence suggests a potential link between HPV infection and BC (26-30). Several studies have investigated the presence of HPV DNA in BC tissues with mixed results. Some studies have reported a higher prevalence of HPV DNA in BC samples compared to normal breast tissue, suggesting a possible role of HPV in breast carcinogenesis (31). However, other studies have failed to find a consistent association between HPV and BC (32). The potential mechanisms of HPV-mediated cancer development have not yet been proven, but it is hypothesized that HPV infection may cause BC through activation of oncogenic pathways, immune dysregulation, or direct integration of viral DNA into the host genome (33-35). In addition, HPV infection may interact with other risk factors, such as hormonal imbalance or genetic predisposition, to increase the risk of BC (36, 37). Understanding the relationship between HPV and BC is important for several reasons. First, if a causal relationship is established, it could have implications for prevention strategies, such as HPV vaccination, which have been successful in reducing the incidence of cervical cancer. Second, the detection of HPV DNA in BC tissues could potentially serve as a biomarker to identify subsets of BC patients who may benefit from targeted therapies or specific therapeutic approaches.
2. Objectives
The aim of this study was to systematically investigate the presence of HPV in women with BC in studies conducted in Iran.
3. Methods
3.1. Study Protocol
This article was written according to the PRISMA guidelines, which describe how to write systematic articles (38).
3.2. Search Strategy
The present study is a systematic study that investigates the presence of HPV in Iranian women with BC. Therefore, Web of Science, Scopus, PubMed, ScienceDirect, EBESCO, Embase, Google Scholar, Magiran, SID, and Irandoc databases were used for searching. The WHO website was also used. The search was updated until 2 August 2023 among scientific articles published in Iranian and non-Iranian journals. Because the present study is related to Iranian women with BC, in addition to international databases, Iranian and Persian language databases were also used. To search the databases of the keywords of BC, HPV, prevalence, ferequency, genotype, cross sectional, seroprevalence, and Iranian women in Persian and English languages were used. Keywords were standardized using the MESH system. Studies consistent with a relationship between HPV and breast cancer have been searched.
3.3. Selection of Articles
First, a list of titles and abstracts of all searched articles in Farsi and English was prepared in ENDNOTE software. This work was done by two researchers independently (AJS and ME) and minor differences were resolved during joint meetings between the two researchers and with the cooperation of MP.
3.4. Inclusion and Exclusion Criteria
The inclusion criteria included: All research articles, thematic relevance, and complete articles. To increase the sensitivity of article selection, minimum inclusion criteria were used. Exclusion criteria included: Lack of relevance, systematic studies, reviews, case reports, animal studies lack of sufficient information, and lack of access to the full text. In the final stage, the content of the articles was examined in terms of methodology. The inclusion and exclusion criteria were controlled by MP researcher.
3.5. Data Extraction
To reduce possible biases, two researchers (ME and MP) independently extracted data from the articles. The data included the place of study, year, name of authors, language of publication, method, number of samples, number of positive samples, percentage of positive samples, and references of articles. Then the content of the articles was given in Table 1.
| Study Place | Year | Authors | Publication Language | Method | No. of Case | Positive Case | P/N % | Ref. |
|---|---|---|---|---|---|---|---|---|
| Rasht | 2023 | Fakour et al. | EN | PCR | 46 | 15 | 32.61 | (39) |
| Tehran | 2023 | Khalilian et al. | EN | Nested PCR | 200 | 24 | 12 | (40) |
| Kermanshah | 2023 | Haghighi et al. | EN | PCR | 90 | 23 | 25.56 | (41) |
| Karaj | 2022 | Hashemnejad et al. | EN | PCR | 503 | 201 | 39.96 | (42) |
| Tehran | 2021 | Golrokh Mofrad et al. | EN | Nested PCR | 59 | 7 | 11.86 | (43) |
| Khuzestan | 2020 | Hosseinpouri et al. | PE | PCR | 40 | 25 | 62.5 | (44) |
| Shiraz | 2019 | Bakhtiarizadeh et al | EN | PCR | 150 | 0 | 0 | (45) |
| Shahrekord | 2019 | Khodabandehlou et al. | EN | PCR | 72 | 35 | 48.61 | (46) |
| Tehran | 2019 | Kazemi Aghdam et al. | EN | Nested PCR | 75 | 0 | 0 | (47) |
| Tehran | 2018 | Ghaffari et al. | EN | Nested PCR | 72 | 4 | 5.56 | (48) |
| Kerman | 2018 | Malekpour Afshar et al. | EN | Real-Time PCR | 98 | 8 | 8.16 | (49) |
| Yazd | 2016 | Doosti et al. | EN | Nested PCR | 87 | 20 | 22.99 | (50) |
| Sanandaj | 2016 | Karimi et al. | EN | PCR | 70 | 2 | 2.86 | (51) |
| Tehran | 2015 | Aghakhani et al. | EN | Nested PCR | 100 | 0 | 0 | (52) |
| Tabriz | 2014 | Ahangar-Oskouee et al. | EN | Nested PCR | 65 | 22 | 33.85 | (53) |
| Karaj | 2014 | Doosti et al. | PE | Nested PCR | 87 | 29 | 33.33 | (54) |
| Isfahan | 2014 | Manzouri et al. | EN | PCR | 55 | 10 | 18.18 | (55) |
| Karaj | 2013 | Hossein et al. | EN | Multiplex PCR | 150 | 52 | 34.67 | (56) |
| Tehran | 2013 | Tahmasebi Fard et al. | PE | Real-Time PCR | 66 | 0 | 0 | (57) |
| Sari | 2012 | Sigaroodi et al. | EN | PCR | 79 | 15 | 18.99 | (58) |
| Mashad | 2009 | Seyedi Alavi et al. | PE | PCR | 50 | 24 | 48 | (59) |
| Golestan | 2009 | Moradi et al. | PE | PCR | 231 | 0 | 0 | (60) |
| Total | 2445 | 516 | 21.10 |
3.6. Data Analysis
In this research, Statistical Package for the Social Sciences (SPSS) software (version 16) was used for statistical analysis of data (Average of samples).
3.7. Quality Assessment
The reviewed studies may have some risks of bias that need to be considered. Firstly, the inclusion and exclusion criteria were controlled by a single researcher, which may introduce bias in the selection process. Additionally, the data extraction was performed independently by two researchers, which could lead to potential biases in the interpretation of the data. Moreover, the methodological differences in the studies, such as the use of different PCR methods, could introduce bias in the comparison of results across different studies. Furthermore, the exclusion of articles due to the inappropriateness of the statement of the working method may have led to the exclusion of relevant studies, potentially introducing selection bias. Therefore, it is important to consider these potential biases when interpreting the findings of the reviewed studies. To reduce bias, the researchers used the risk of bias in non-randomized studies (ROBINS) strategy (61, 62) and the participation of all three researchers (AJS, ME, and MP) in the final analysis.
4. Results
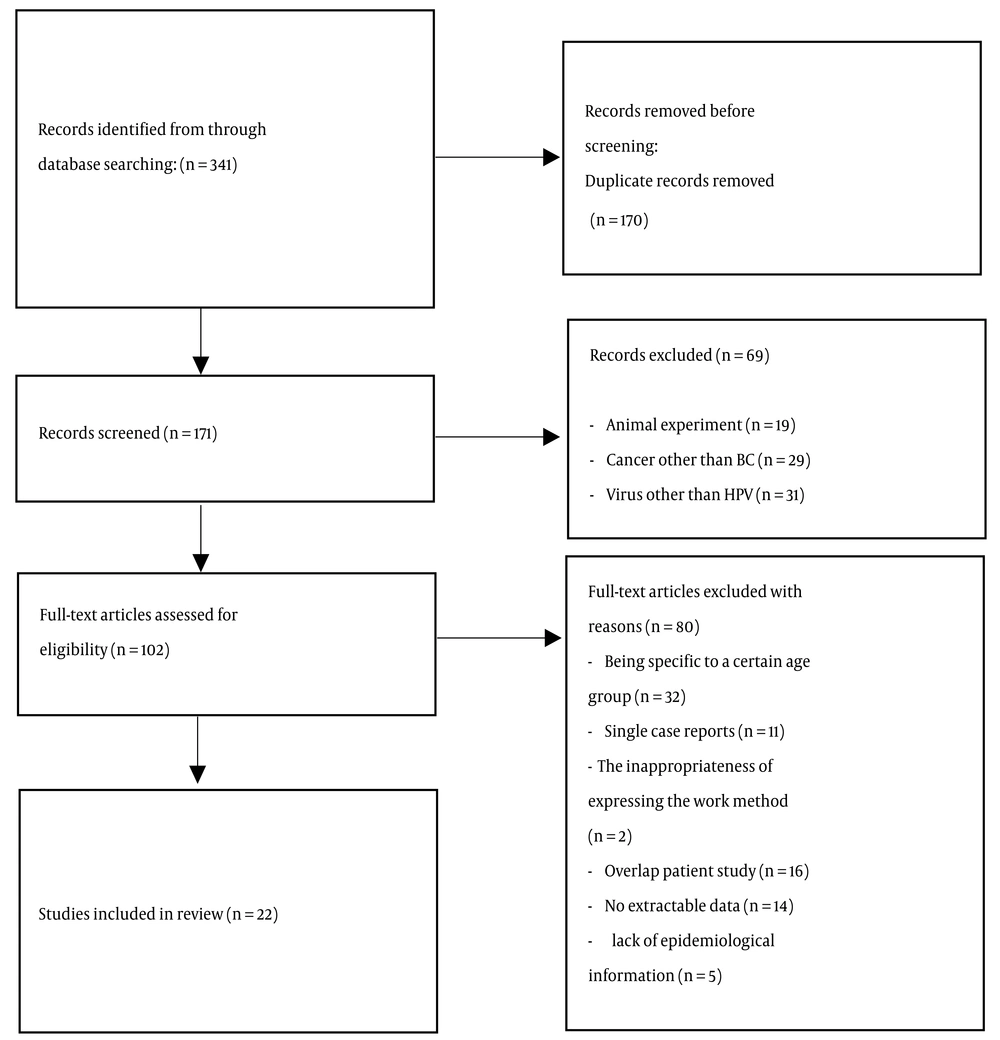
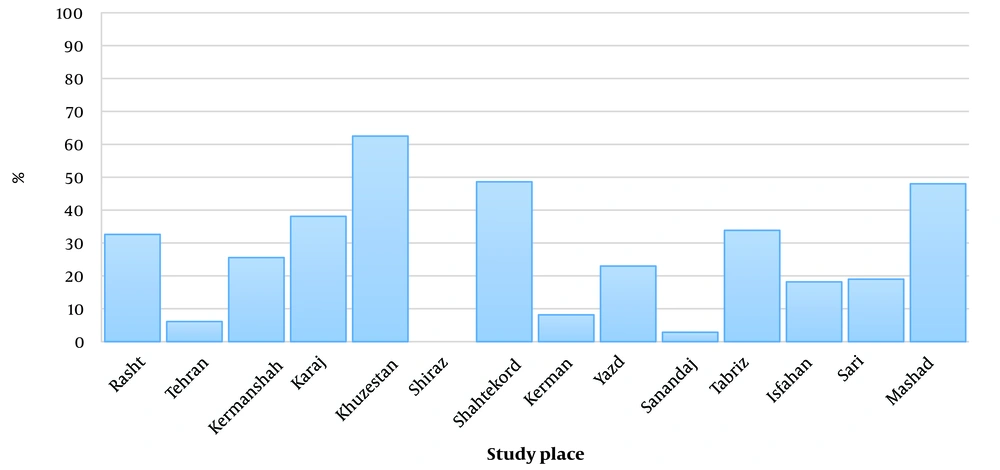
The initial search based on the prepared checklist included 341 articles, of which 170 articles were removed due to duplication. After the initial review of articles (171 articles), 102 related articles were selected and reviewed. 32 articles due to being specific to a certain age group, 16 articles due to overlapping with other studies, 14 articles due to lack of extractable data, 11 articles due to single case reports, 5 articles due to lack of epidemiological information, and 2 articles. They were excluded from the review process due to the inappropriateness of the statement of the working method. Finally, 22 published articles 5 (22.73%) articles in Persian language and 17 (77.27%) articles in English language) were reviewed and analyzed (Figure 1). In total, from 22 articles with 2445 BC samples, 21.10% (516 samples) were HPV positive. Khuzestan, Shahrekord, Mashhad, and Karaj had the highest number of HPV-positive cases among women with BC with odds ratio of 62.5%, 48.61%, 48%, and 38.11%, respectively (Figure 2). Among them, Golestan and Shiraz had a chance ratio of zero percent. PCR (50%), nested PCR (36.36%), real-time PCR (9.10%), and multiplex PCR (4.54%) were the most investigated methods in the studies. Out of 2445 BC samples, the most distribution was related to the cities of Karaj (740 samples), Tehran (572 samples), Golestan (231 samples), Shiraz (150 samples), Kerman (98), Kermanshah (90 samples), Yazd (87), Sari (79 samples), Shahrekord (72 samples), Sanandaj (70 samples), Tabriz (65 samples), Isfahan (55 samples), Mashhad (50 samples), Rasht (46 samples), and Khuzestan (40 samples).
5. Discussion
This systematic review was conducted with the aim of investigating the presence of HPV infection in Iranian women with BC. This study analyzed related studies conducted in Iran and evaluated the prevalence of HPV infection in BC tissues in Iranian women. From 22 related articles, a total of 2445 cases of BC were identified, and the overall prevalence of HPV in Iranian women with BC was 21.10%. This suggests that HPV infection may be associated with a higher risk of BC in Iranian women. Therefore, the findings of the systematic review show the potential relationship between HPV infection and BC in Iranian women. Several studies included in this review reported the presence of HPV DNA in BC tissues, indicating the possible involvement of HPV in breast carcinogenesis (33, 63). In the Middle East, studies have shown that HPV is present in BC tissues in Iraqi, Pakistani, Syrian, Turkish, and Qatar women (64-68). However, there are also studies from Tunisia that failed to detect HPV in BC tissues (69). The prevalence of HPV infection in BC may be different in different countries of the Middle East region. In Africa, countries such as Morocco, Algeria, Nigeria, and Congo reported the presence of HPV in women with BC (70-74). In their 2011 systematic review, Li et al. showed that 32.42%, 12.91%, 16.67%, 20%, and 42.11% of HPV-positive BC cases occurred in Asia, Europe, South America, North America, and Oceania (75). However, it is important to note that the results of the included studies were not consistent. Some studies reported a higher prevalence of HPV DNA in BC samples compared to normal breast tissue, while others found no significant association. These discrepancies may be attributed to variations in study design, sample size, diagnostic methods, and patient characteristics (63). The potential mechanisms by which HPV may contribute to the development of BC are not yet fully understood. It is hypothesized that HPV infection may activate oncogenic pathways, induce immune dysfunction, or directly integrate viral DNA into the host genome, leading to BC development (33-35). However, more research is needed to clarify the underlying mechanisms and establish a definitive link between HPV and BC. The findings of this systematic review have important implications for the prevention and management of BC in Iran. If the relationship between HPV and BC is established, it could have implications for HPV vaccination strategies, similar to the successful implementation of HPV vaccination to prevent cervical cancer. In addition, the detection of HPV DNA in BC tissues could potentially serve as a biomarker to identify a subset of BC patients who may benefit from targeted therapies or specific therapeutic approaches (76-79). It is worth mentioning that this systematic review is specifically focused on Iranian women with BC. Therefore, the findings may not be generalizable to other populations. Further research, including larger-scale studies and diverse populations, is needed to confirm the findings and determine the global relevance of the association between HPV infection and BC.
5.1. Conclusions
The present study shows the presence of HPV infection in Iranian women with BC and the potential relationship between HPV and BC in this population. Several studies included in this review reported the presence of HPV DNA in BC tissues, indicating a possible role of HPV in breast carcinogenesis. However, the results of the included studies were not consistent, and further research is needed to confirm this association and understand the underlying mechanisms. The findings of this systematic review have important implications for the prevention and management of BC in Iran. If a causal relationship between HPV and BC is established, it could have implications for HPV vaccination strategies and the development of targeted therapies for a subset of BC patients. However, it is important to note that the review specifically focused on Iranian women and more research in different populations is needed to confirm the findings and determine the universality of the association between HPV infection and BC.