1. Background
Over the past few decades, with the advancement of technology, machine life, improving the level of public and individual health, and increasing life expectancy, the elderly population has been increasing in various societies (1). Nowadays, the growing population of elderly people and its consequences is one of the major population issues that the countries of the world are dealing with (2). Regular physical activity prevents cardiovascular diseases. Cardiovascular protective systems will deteriorate and deteriorate with aging, while in order to have a stable cardiovascular system, the existence of protective systems is very necessary and important (3). Recently, the terms sarco-osteopenia, sarco-osteoporsis, sarcopenic obesity (SO) and ostosarcopenic obesity (OSO) have been noticed by scientists. In fact, these terms will provide a link between decreased muscle and bone mass and increased fat mass in the elderly (4).
Insulin resistance includes a decrease in the sensitivity of muscles and adipose tissue to insulin and a decrease in the ability of the liver to consume glucose. In fact, insulin resistance is considered a risk factor for type 2 diabetes and cardiovascular diseases (5).
Although the hyperinsulinemic clamp technique is a gold standard method for measuring insulin resistance, its use is limited by human resources and medical costs, as well as ethical concerns. Therefore, a simple, reliable and renewable index is needed for measurement (6).
The research results show that triglyceride-glucose (TyG) and triglyceride-glucose related to body mass index (Tyg-BMI) are very good markers for diagnosing insulin resistance and are recognized as a vital pathological mechanism (7).
It has been shown that the triglyceride-glucose index (TyG) was measured by hyperinsulinemia and hyperinsulinemia clamp studies. In addition, TyG is an alternative marker to estimate the homeostasis model assessment index (HOMa-IR) in healthy people and shows higher sensitivity and characteristics compared to hyperinsulinemia for the diagnosis of insulin resistance. The advantage of using the TyG index in identifying insulin resistance has been reported in many studies, and with these findings, a very efficient alternative has been created to measure and diagnose insulin resistance, which can be easily used in the clinical environment (8).
Cardiovascular diseases, including atherosclerosis, high blood pressure and heart attack, have a direct relationship with aging (9)
One of the most important problems in the field of health and treatment is heart failure, and its occurrence is associated with high morbidity and mortality and significant care and treatment costs (10). Many studies have been conducted in the field of identifying the best predictors of cardiovascular diseases. However, it has been observed that in some people, despite the normality of traditional risk factors (especially blood lipoproteins), they have suffered from cardiovascular disorders (11). Therefore, today researchers intend to discover indicators that predict the risk of cardiovascular diseases more accurately and sensitively, and considering that elderly people are not able to perform heavy sports, it is better to do light sports such as slow running, walking and swim (3).
2. Objectives
In this research, we aim to investigate the effect of 12 weeks of Nordic walking on a new cardiovascular index, including galectin-3 levels and plasma atherogenic index and some metabolic markers such as triglyceride-glucose index (TyG). Let's examine the triglyceride-glucose index-body mass index (TyG-BMI) and the triglyceride-glucose index-waist circumference (TyG-WC).
3. Methods
3.1. Subjects
This research is applied, semi-experimental and double blind experimental design. Twenty subjects were selected in an accessible and purposeful manner and were divided into 2 Nordic walking training groups and a control group (10 people in each group) in a simple random manner. The size of the statistical sample in each group was determined by using MedCalc version 10.0.2.0 software, considering the significance level of 5% and the power of the test of 0.8, about 15 people.
The subjects of this research included elderly men with with Mean age of 60 to 80 years and Mean weight of 96 ± 3 who had osteosarcopenic obesity.
The inclusion criteria for the study included elderly men with Mean age of 60 to 80 years who had osteosarcopenic obesity with a body mass index (equal to and above 30) and body fat percentage (equal to and above 30) and had no history of obesity in the past year. They did not have regular sports activities. The indicators for leaving the research included not regularly participating in training sessions, smoking, suffering from infectious and cardiovascular diseases, having type 1 diabetes, and having medical and orthopedic restrictions and other problems to participate in physical activity. After registering the candidates and before entering the study, all the participants were familiarized with the purpose and possible risks of the research, and the necessary information for the subjects was fully explained and presented.
3.2. Nordic Walking Exercise Protocol
Nordic walking exercise was performed for 12 weeks and 3 days a week. The intensity of the exercises in each session was 50 to 75% of the maximum heart rate and the duration of each exercise session was 60 minutes. The training protocol started with an intensity of 50% of the maximum heart rate and every two weeks the intensity of the activity was increased by 5% and finally reached 75% of the maximum heart rate in the last two weeks. In Nordic walking exercise, special walking sticks were used. One week before the start of the exercises, how to use these hand sticks was explained to the subjects (12). In each training session, first the subjects did stretching and warming up for 10 minutes, then they started walking for 40 minutes. Hand sticks are used in pairs and the height of the sticks was adjusted for each person separately. At the end, the subjects cooled down their bodies for 10 minutes (Table 1).
| Weeks | Warm Up and Cool Down (Minutes) | Training Period (Minutes) | Intense Training (%) | The Total Duration of the Activity (Minutes) |
|---|---|---|---|---|
| 1 - 2 | 20 | 40 | 50 | 60 |
| 3 - 4 | 20 | 40 | 55 | 60 |
| 5 - 6 | 20 | 40 | 60 | 60 |
| 7 - 8 | 20 | 40 | 65 | 60 |
| 9 - 10 | 20 | 40 | 70 | 60 |
| 11 - 12 | 20 | 40 | 75 | 60 |
Nordic Walking Exercise Protocol
3.3. Evaluation of Research Variables
The measurement of anthropometric and serum indicators of the subjects was done in the pre-test phase, one week before the start of the process of implementing the training protocol. In addition, the measurements of the post-test stage were performed about 48 hours after the last training session.
To evaluate the atherogenic index of plasma (AIP) using serum levels of TG and HDL-c indices and using the following formula was used (Collaboration, 2005).
AIP=Log (TG/HDL-c)
In the AIP equation, TC/HDL-c and APOB/APOA-1 ratios and all invasive factors were measured in mg/dL.
In order to evaluate the TyG index, we first measured the serum levels of triglycerides and fasting glucose in the serum, and by putting these values in the following formula, we obtained the amount of TyG: TyG= (fasting Triglycerides mg/L ×fasting Glucose mg/dL)/2
To evaluate the TyG-BMI index, the following formula was used:
TyG-BMI = TyG index × BMI
To evaluate the TyG-WC index, the following formula was used:
TyG-WC = TyG index × WC
3.4. Statistical Analysis Methods
Descriptive statistics were used to describe the data and draw tables in the form of mean and standard deviation, Shapiro-Wilk test were used to check the normality of the data, and Levene's test was used to evaluate the homogeneity of variances. Analysis of variance with repeated measures and Tukey's post hoc test were used. All data were analyzed at a significance level of 0.05 using SPSS-22 software.
For the statistical analysis of variables, analysis of variance with repeated measures and Tukey's post hoc test were used. All data were analyzed at a significance level of 0.05 using SPSS-22 software.
4. Results
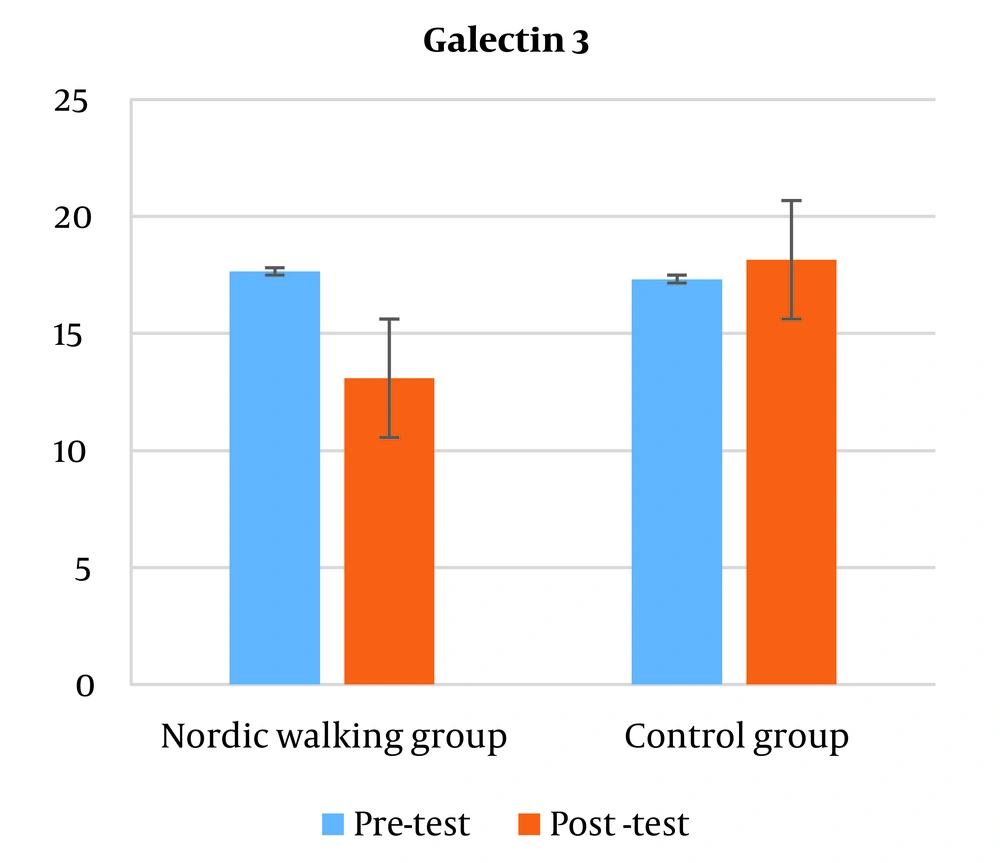
The results of analysis of variance with repeated measurements showed that there is a significant difference in the plasma levels of galectin 3 between the two groups of control and Nordic walking (P = 0.001). The independent t-test results showed that plasma levels of galectin-3 decreased significantly in the Nordic walking group compared to the control group (Figure 1).
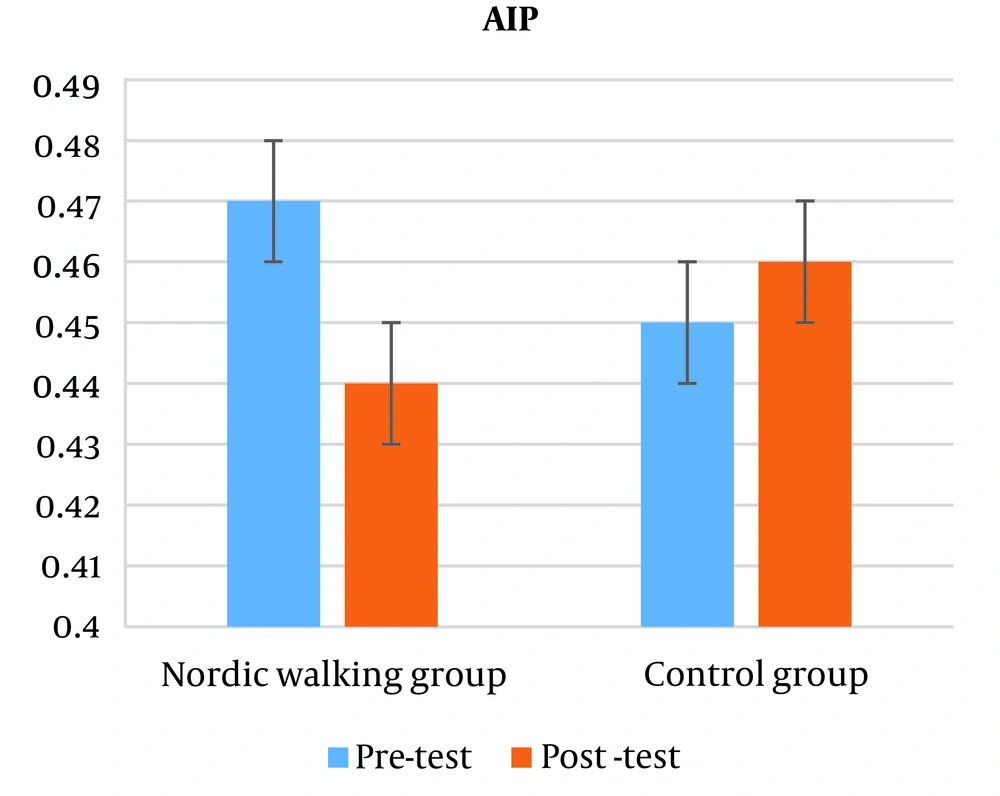
The results showed that there is no significant difference in the atherogenic index of plasma (AIP) between the two control groups and Nordic walking (P = 0.458). Also, based on the independent t-test results, no significant difference was observed in the atherogenic index of plasma (AIP) between the two groups (P = 0.352) (Figure 2).
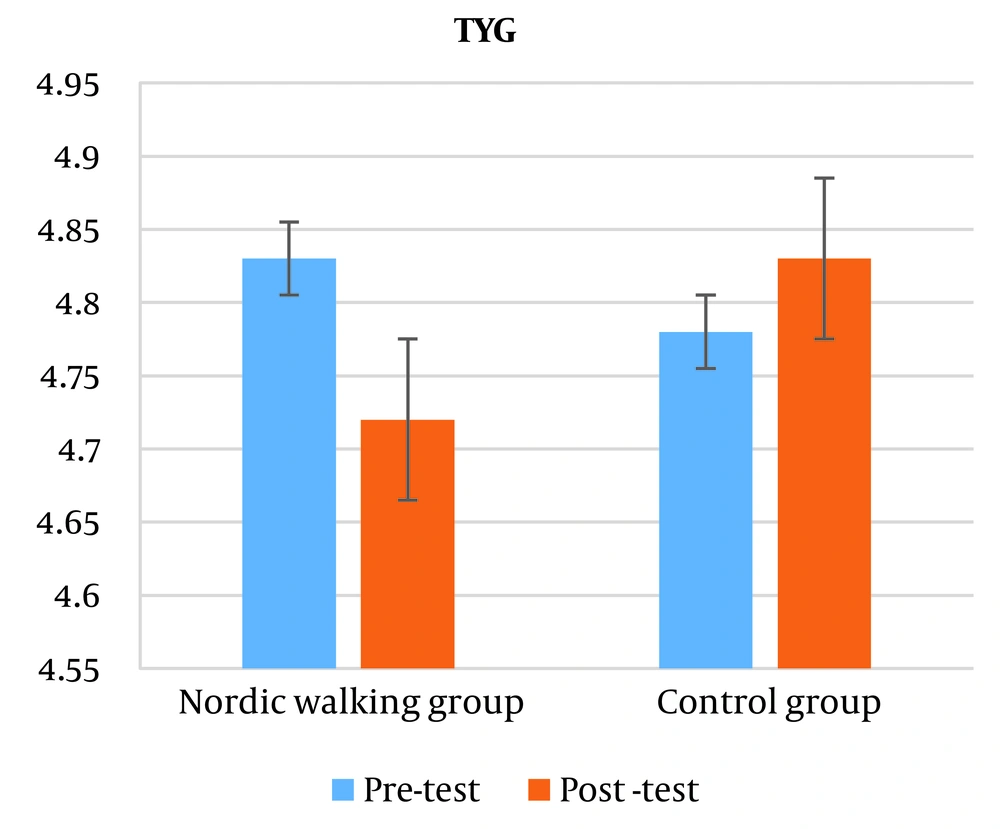
The results did not show a significant difference in the triglyceride glucose index (TyG) of the subjects (P = 0.274). Also, based on the results of the independent t-test, no significant difference was observed between the two groups in the triglyceride glucose (TyG) index (P = 0.274) (Figure 3).
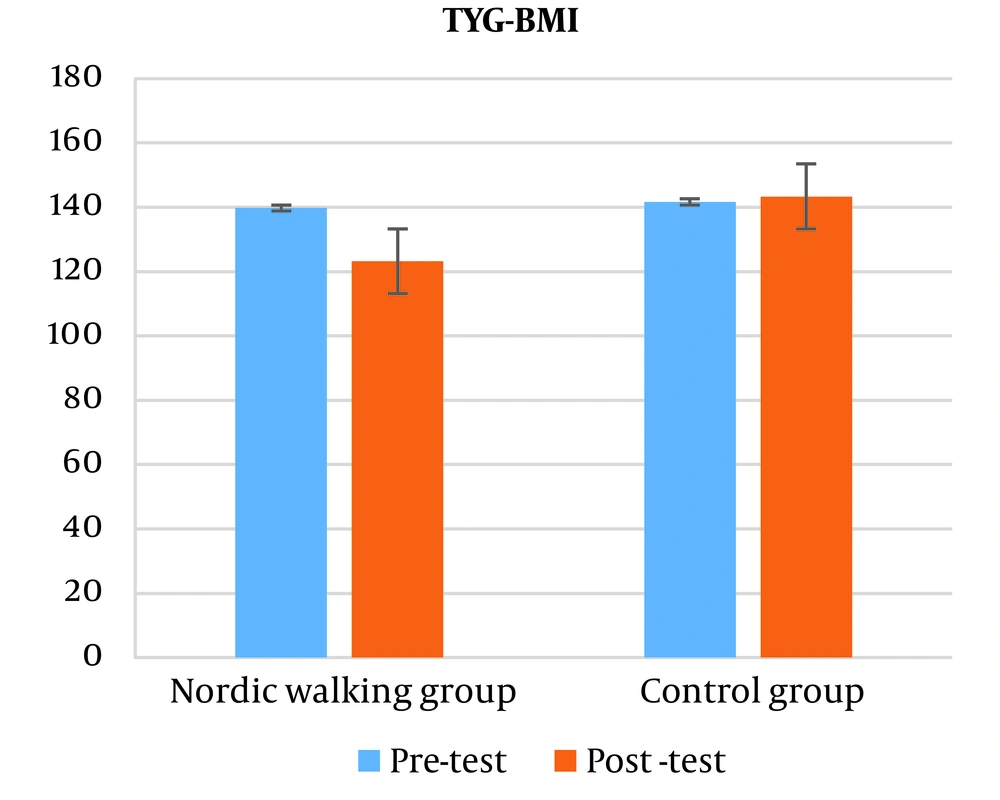
The results showed that there is a significant difference in triglyceride-glucose-body mass index (TyG-BMI) between the two control and Nordic walking groups (P = 0.001). The independent t-test results showed that the triglyceride-glucose-body mass index (TyG-BMI) decreased significantly in the Nordic walking group compared to the control group (P = 0.001) (Figure 4).
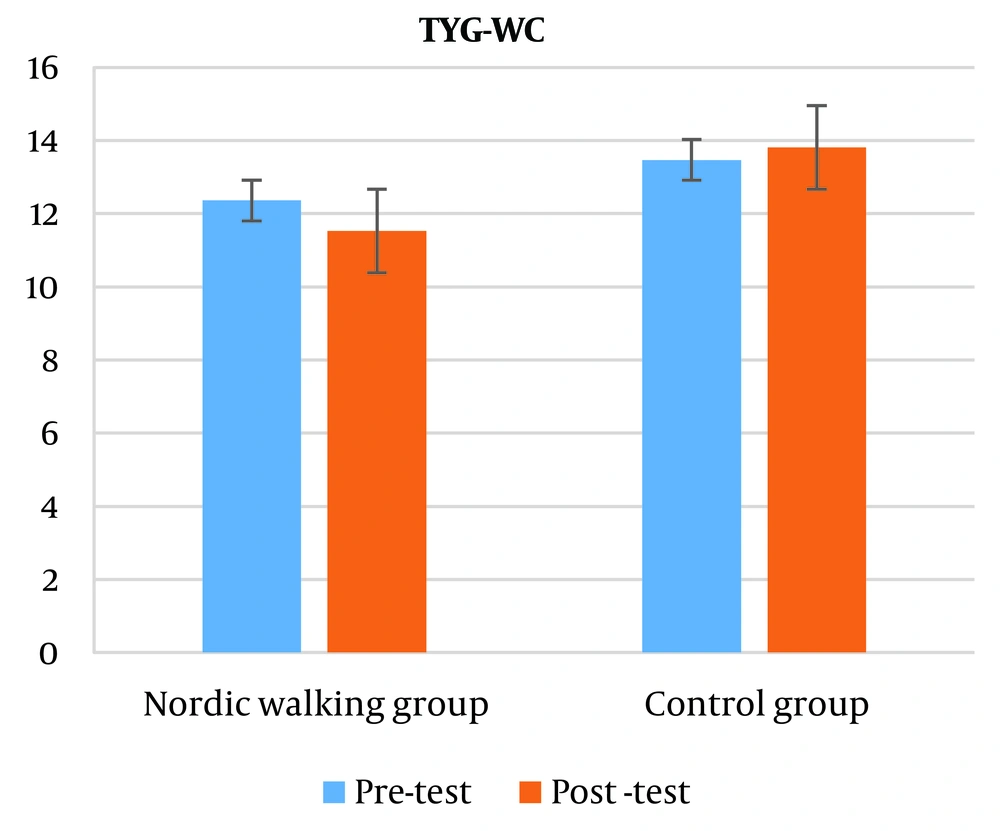
The results showed no significant difference in the triglyceride-glucose-waist circumference index (TyG-WC) of elderly men with osteosarcopenic obesity (P = 0.419). Also, based on the results of the independent t-test, no significant difference was observed between the two groups in the triglyceride-glucose-waist circumference (TyG-WC) index (P = 0.126) (Figure 5).
5. Discussion
The results of this research showed that the plasma levels of the cardiovascular biomarker Galectin 3 in the Nordic walking group had a significant decrease compared to the control group. Galectin 3 is a biomarker secreted by activated cardiac macrophages that is involved in cardiac inflammation and fibrosis and is strongly associated with the development of heart failure (13).
The results of studies have shown that the expression of galectin 3 indicates many processes related to cardiac abnormalities, including myofibroblast proliferation, fibrogenesis, tissue repair, inflammation, and ventricular changes (14). Increased levels of galectin-3 are significantly associated with the risk of death from acute and chronic cardiac abnormalities (14).
In addition, galectin-3 levels are high in obese and diabetic individuals and have a positive relationship with body mass index, which is involved in the onset and progression of metabolic disorders (15).
One of the important points is that the main source of galectin-3 secretion during exercise is the skeletal muscle, not the myocardium. And it increases with intense physical activity (16). Exercise may reduce galectin-3 levels by improving body composition, especially visceral obesity (waist circumference index). Visceral fat may contribute to higher galectin-3 levels in obese people, it appears that galectin-3 levels are higher in people with higher visceral fat than subcutaneous fat (17). In the present study, 12 weeks of Nordic walking training had the same effect on the atherogenic index of plasma (AIP) in both training and control groups. Atherogenic index of plasma (AIP) is a relatively new lipoprotein index and is obtained from the logarithm of the TG/HDL-c ratio and indicates the presence of small and dense LDL particles, which can be used as a suitable predictive measure for coronary heart disease (18). In fact, the low ratio of TG/HDL-c indicates that the LDL particles are large and non-atherogenic. Also, high TG/HDL-c ratio indicates more small, dense and atherogenic LDL particles. This index determines the path of cholesterol transfer inside the vessels and indicates the balance between the actual concentration of plasma TG and HDL-c (19). With the increase of TG/HDL-c ratio, HDL particles tend towards smaller sizes, and therefore a high ratio indicates the possibility of stopping HDL-c and weakening the reverse transport of cholesterol (20). Physical activity is associated with more oxidation of lipids; an increase in the muscles' need for energy causes more access to fatty acids. Most of these fatty acids are provided by lipolysis of adipose tissue 2 to 3 times more than in the resting state. This process takes place indirectly by increasing beta-adrenergic stimulation. In addition, the percentage of re-esterification of fatty acids is reduced by half. Also, intense sports activity increases blood flow in fat tissue up to 2 times and in skeletal muscles up to 10 times. This increase in blood flow may prevent the toxic effects of lipid accumulation and help improve atherogenic index values (21).
One of the pathways that change HDL-c levels and improve cholesterol transport is the reverse cholesterol transport pathway, in which excess cholesterol is largely collected from the peripheral tissues, especially the macrophages of the arterial wall, and transferred to the liver (22). The results of the studies show that one of the activators of this pathway is physical activity, which can increase lymphocytes and affects the expression of ABCA1 (ATP-Binding cassette transporter protein) and the formation of HDL-c (23).
Another mechanism that justifies the effect of exercise activity on the expression of ABCA1 and HDL-c is the role of the liver receptors RXR (retinoid X receptor) and LXR (liver-X- receptor ) , which have been shown to be involved in regulating the expression of genes that control fat and glucose metabolism (23). We can also mention the role of PPAR receptors (Peroxisome proliferator-activeted receptir) in cholesterol metabolism, which regulates the metabolism of fats (24).
Also, in the present study, doing 12 weeks of Nordic walking training had the same effect on triglyceride-glucose (TyG) and triglyceride-glucose-waist circumference (TyG-WC) indices in both training and control groups, but the triglyceride index Glyceride glucose-body mass (TyG-BMI) in the Nordic walking group has significantly decreased compared to the control group.
Based on the researches, lipolytic activity is heterogeneous in different stores of fat tissue (subcutaneous or intra-abdominal). Visceral adipose tissue is the most active storage of adipose tissue in terms of lipolytic activity (25). Despite the fact that visceral fat has more lipolytic activity and is affected by exercise faster than other stores, it has a smaller contribution in providing fatty acids for muscle oxidation during exercise. Therefore, it seems that most of the fatty acids that enter the blood circulation are extracted from the subcutaneous fat tissue. Lipolytic activity in different parts of the subcutaneous fat of the body is also heterogeneous. The results of the studies show that with the increase in the intensity of exercise, the contribution of lower body fat tissue in providing free fatty acids increases by only about 10% (26). Exercise training will increase the ability of skeletal muscles to use lipids against glycogen, which mechanism will reduce plasma lipid levels. In fact, the process of skeletal muscle utilization of lipids may include an increase in the levels of cholesterol acyl lecithin transfer enzyme (LCAT), the enzyme responsible for transferring the ester group from high density lipoprotein cholesterol, which increases due to exercise and increases the activity of lipoprotein lipase and may be dependent on the energy consumed due to the type of sports activity (27).
In this research, despite the fact that the TyG index shows a decrease in the post-test compared to the pre-test, this decrease is not significant. Maybe the reason for the lack of significance is due to the age of the subjects, the degree of obesity and the type of obesity. It has been reported that the TyG index is suitable for estimating the HOMA-IR index in healthy subjects, and in subjects with chronic inflammation, this index is not a suitable criterion for HOMA-IR estimation (28).
Regarding the significant decrease in the TyG-BMI index, it should be said that due to the direct relationship between BMI and body weight and the weight loss of the subjects of this research, therefore, the BMI of the subjects also decreased in the post-test, and this decrease in the BMI of the subjects caused a significant decrease in the TyG index. -BMI, which shows the effect of Nordic walking exercises in the elderly with osteosarcopenic obesity. However, the TyG-WC index is another index that was examined in this research and despite the decrease of this index in the post-test compared to the pre-test, this decrease was not significant and it seems that walking exercises as shown in The TyG index did not have an effect, and there was no significant effect on the waist circumference index (WC) of the research subjects.
5.1. Conclusions
It seems that performing Nordic walking exercises reduces cardiovascular risks in people. Considering the impact of using a walking stick on the intensity of training in Nordic walking, it is better for people who do walking to maintain their health to use these devices to improve the efficiency of training.
5.2. Limitations
The limitations of the present research include: lack of control over the genetic characteristics of the subjects; lack of accurate control of the amount of activities outside the hours of the subjects' training schedule; Failure to control the level of spirit and motivation of the subjects to implement the training program.