1. Background
Acinetobacter baumannii is a species of Gram-negative bacteria that is commonly found in the environment, particularly in soil and water. It is an opportunistic pathogen that can cause a range of infections in humans, especially in healthcare settings such as hospitals and long-term care facilities (1). Acinetobacter baumanniiis known for its ability to develop multidrug resistance, making it challenging to treat and control infections caused by this bacterium. It can cause infections such as pneumonia, bloodstream infections, urinary tract infections, and wound infections, particularly in individuals with weakened immune systems or those who have undergone invasive medical procedures (2). Finding anti- A. baumannii compounds is of great importance due to the increasing prevalence of multidrug-resistant strains of this bacterium (3). Acinetobacter baumanniiinfections are particularly problematic in healthcare settings, causing high morbidity and mortality rates, especially among vulnerable patient populations (4). Developing effective compounds that can combat A. baumannii infections is crucial for improving patient outcomes, reducing the spread of infections, and preserving the effectiveness of existing antibiotics (5). AAcinetobacter baumanniiis known for its ability to develop resistance to multiple antibiotics, including those commonly used in clinical practice. Some of the important resistance genes found in A. baumannii are: OXA-type beta-lactamase genes (6), TEM and SHV beta-lactamase genes (7), AmpC beta-lactamase genes (8), Aminoglycoside-modifying enzyme genes (9), Fluoroquinolone resistance genes (10) and Efflux pump genes (11). The OXA-23 gene in A. baumannii is a genetic element that encodes for a type of beta-lactamase enzyme known as OXA-23. This enzyme belongs to the class D beta-lactamases and is responsible for conferring resistance to beta-lactam antibiotics, including carbapenems (12). Carbapenems are potent antibiotics commonly used as a last resort for treating multidrug-resistant bacterial infections. The presence of the OXA-23 gene in A. baumannii strains allows them to produce the OXA-23 enzyme, which can degrade and inactivate these antibiotics, leading to treatment failure and the spread of carbapenem-resistant A. baumannii strains. The OXA-23 gene is a significant factor contributing to the multidrug resistance and clinical challenges associated with A. baumannii infections (13). Flavonoids are a diverse group of naturally occurring compounds widely distributed in the plant kingdom. They serve various biological functions and exhibit numerous health benefits like Antioxidant Activity, Anti-inflammatory properties, Cardiovascular Health, Cancer Prevention, Anti-Microbial Activity, Neuroprotective Effects and Anti-Allergic Actions (14). Flavonoids have shown antibacterial activity against various bacterial species. They can inhibit the growth and proliferation of bacteria by disrupting their cell membranes, inhibiting essential enzymes, or interfering with bacterial DNA replication (15). Flavonoids have been studied for their effectiveness against both Gram-positive and Gram-negative bacteria, including antibiotic-resistant strains such as methicillin-resistant Staphylococcus aureus and multidrug-resistant Escherichia coli (16). The antimicrobial effects of flavonoids are attributed to their ability to interfere with microbial cell structures and metabolic processes. They can disrupt the integrity of the cell membrane, leading to leakage of cellular contents and eventual cell death. Flavonoids can also inhibit specific microbial enzymes, such as DNA gyrase and beta-glucosidase, which are essential for microbial growth and survival (17). Oxadiazole compounds are a class of organic compounds that contain a five-membered ring consisting of three carbon atoms, one oxygen atom, and one nitrogen atom. These compounds have gained attention in medicinal chemistry due to their diverse biological activities, including antibacterial effects. Oxadiazole compounds have demonstrated significant antibacterial activity against a wide range of bacterial strains (18). They have been studied for their effectiveness against both Gram-positive and Gram-negative bacteria, including drug-resistant strains. Oxadiazole derivatives have shown potential in inhibiting bacterial growth and disrupting bacterial cell functions. The antibacterial effects of oxadiazole compounds are attributed to their ability to interfere with essential bacterial processes. They can disrupt bacterial cell membranes, inhibit vital enzymes, interfere with DNA replication, and disrupt bacterial biofilm formation. By targeting multiple bacterial pathways, oxadiazole compounds can exert potent antibacterial effects (19). Molecular docking plays a crucial role in drug discovery and design, allowing researchers to optimize and prioritize compounds for further experimental validation, ultimately accelerating the development of novel therapeutics and providing a cost-effective approach to identify potential leads.
2. Objectives
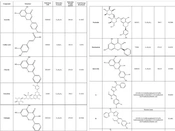
The purpose of this study was to evaluate the In vitro anti-A. baumannii effects of oxadiazole derivatives (1,3,4-oxadiazole in core, and dichlorophenyl, dibromophenyl, difluorophenyl and dimethoxyphenyl are functional groups) and investigate the In silico effects of flavonoid compounds (Acacetin, Caffeic acid, Chrysin, Eriocitrin, Galangin, Kaempferol, Myricetin, Narirutin, Pinobanksin and Quercetin) and oxadiazole derivatives against OXA-23.
3. Methods
3.1. In Vitro
All derivatives of oxadiazole from our pervious study were resynthesizing (20). Solubilizations of the new compounds were done by dimethylsulfoxide (DMSO) solvent at a concentration of 1mg/ml in order to check the antibacterial activity. Also, the pure powder of imipenem (IPM) antibiotic manufactured by Sigma Company was prepared and used as a control at a concentration of 1 mg/mL according CLSI guidelines. The antibacterial activity of the synthesized compounds were investigated by agar well diffusion method, Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) methods against A. baumannii. The nutrient agar/broth culture medium was prepared according to the brochure. Bacterial strain was prepared from Iranian Research Organization for Science and Technology (IROST) (A. baumannii PTCC1919). The bacterial sample was removed from lyophilization and prepared for testing. The bacterial sample was cultured as grass and uniformly on the culture plate. The required wells were created on the culture medium (5 mm) by sterilized Pasteur pipette and the wells were filled with solutions prepared from oxadiazole derivatives and the control sample. The plates were placed in an incubator at 37°C for 24h. After 24h, the plates were examined in terms of determining the presence of no growth halo. Then the inhibitory effect was determined using the tube dilution determination, to check the antibacterial effect of the derivatives, successive dilutions of each derivative (15.625 - 1000 µg/mL) were prepared in nutrient broth culture medium. After 24 h and 37°C, the tubes were visually checked for turbidity, and the highest dilution that inhibited the growth of bacteria (no turbidity) was considered as the MIC. Non-growth samples were cultured on nutrient agar medium and the highest dilution causing no growth was reported as MBC (21).
3.2. In Silico
The crystallized structure of 10 flavonoid compounds as ligands were downloaded from the PubChem database in SDF format. The 2D Structures and names, PubChem ID, Molecular Formula, Molecular Weight and Total Energy are reported in Figure 1. The structures were converted from .SDF to .pdb by ChemDraw 19.01 software. Also, 2D structures of oxadiazole derivatives were draw by ChemDraw 12.0.2.1076 software, Using Chem3Dpro 12.0.2.1076 software to normalize the ligands with MM2 method (The MM2 force field is a computational method used to calculate the potential energy and geometry of molecules.) (22). Crystallized structure of OXA-23 (PDB ID: 4K0X, https://www.rcsb.org/structure/4K0X) of A. baumannii were downloaded from Protein Data Bank (PDB) with resolution of 1.61°A (angstrom). Gastiger charge and polar hydrogens added with AutoDockTools-1.5.6 software. All water molecules and ligands were deleted from main chains of each structure. Grid box for 4K0X = 62 × ((1E,2E)-3-62 × 62 with spacing 3°A. The docking process of 10 flavonoid compounds and oxadiazole derivatives to 4K0X binding site were performed using AutoDockVina software and Discovery Studio 4.5. Ligand and junction interactions were investigated and analyzed by Discovery Studio 4.5 Client software (23).
4. Results
4.1. In Vitro
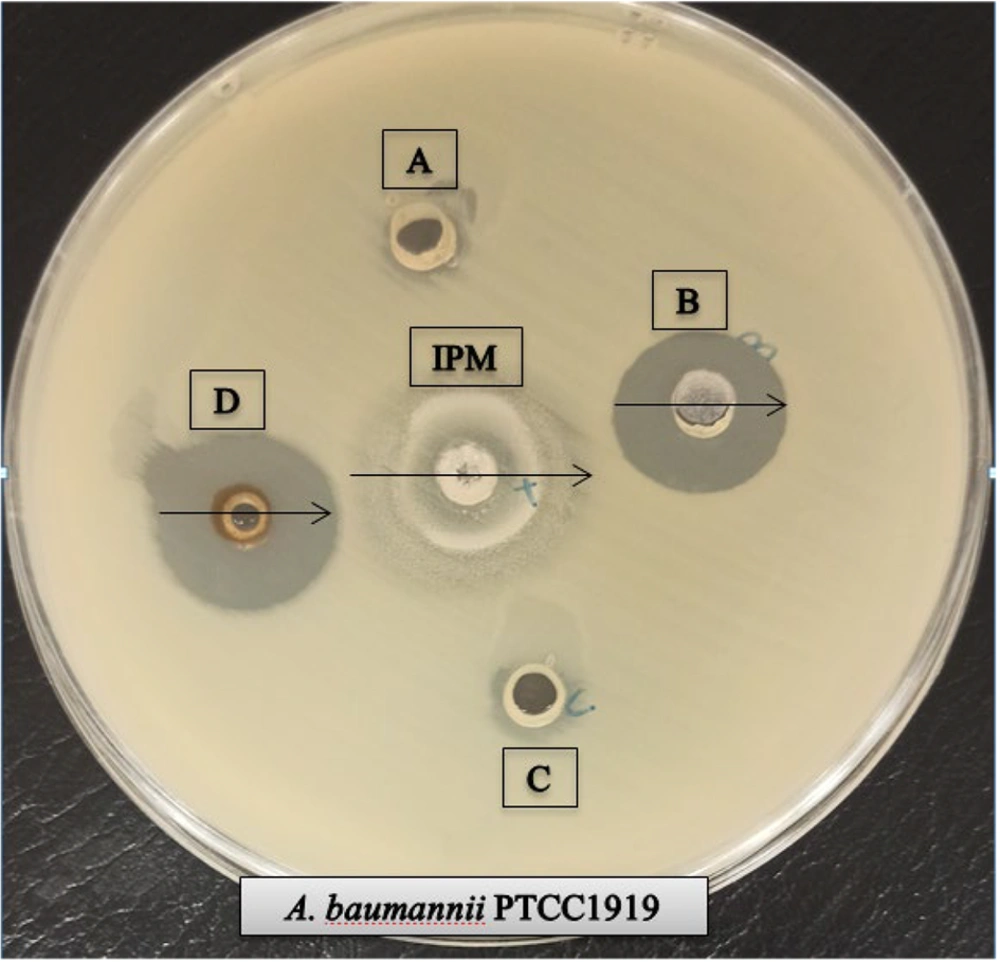
Antibacterial activity of the prepared oxadiazole derivatives (A-D) moieties were evaluated (1 mg/mL). The diameters of inhibition zone (IZ) for each compound are reported in Table 1.
| Compounds | IZ (mm) | (µg/mL) | |
|---|---|---|---|
| MIC | MBC | ||
| A | 7 ± 0.5 | 1000 | 1000 |
| B | 25 ± 0.5 | 62.50 | 125 |
| C | 9 ± 0.5 | 1000 | 1000 |
| D | 26 ± 0.5 | 62.50 | 125 |
| IPM | 30 ± 0.5 | 15.625 | 31.25 |
Compounds B ((1E,2E) -3- (3,4-dibromophenyl) -2-(5-((S) -hydroxy(pyridin-2-yl)methyl) -1,3,4 -oxadiazol-2-yl) -N-methylacrylimidic acid) with dibromophenyl group and D ((1E,2E) -3 -(3,4 -dimethoxyphenyl)-2-(5-((S)-hydroxy(pyridin -2-yl) methyl) -1,3,4-oxadiazol- 2-yl)- N. methylacrylimidic acid) with dimethoxyphenyl group showed power antibacterial activity compared to other compounds (Figure 2).Compounds A and C also showed no notable antibacterial activity. Compound D with IZ = 26 ± 0.5 mm, MIC = 62.50 µg/mL and MBC = 125 µg/mL showed the best performance compared to other synthesized compounds against A. baumannii, Also compound B showed similar effects with the Compound D (Figure 3).
4.2. In Silico
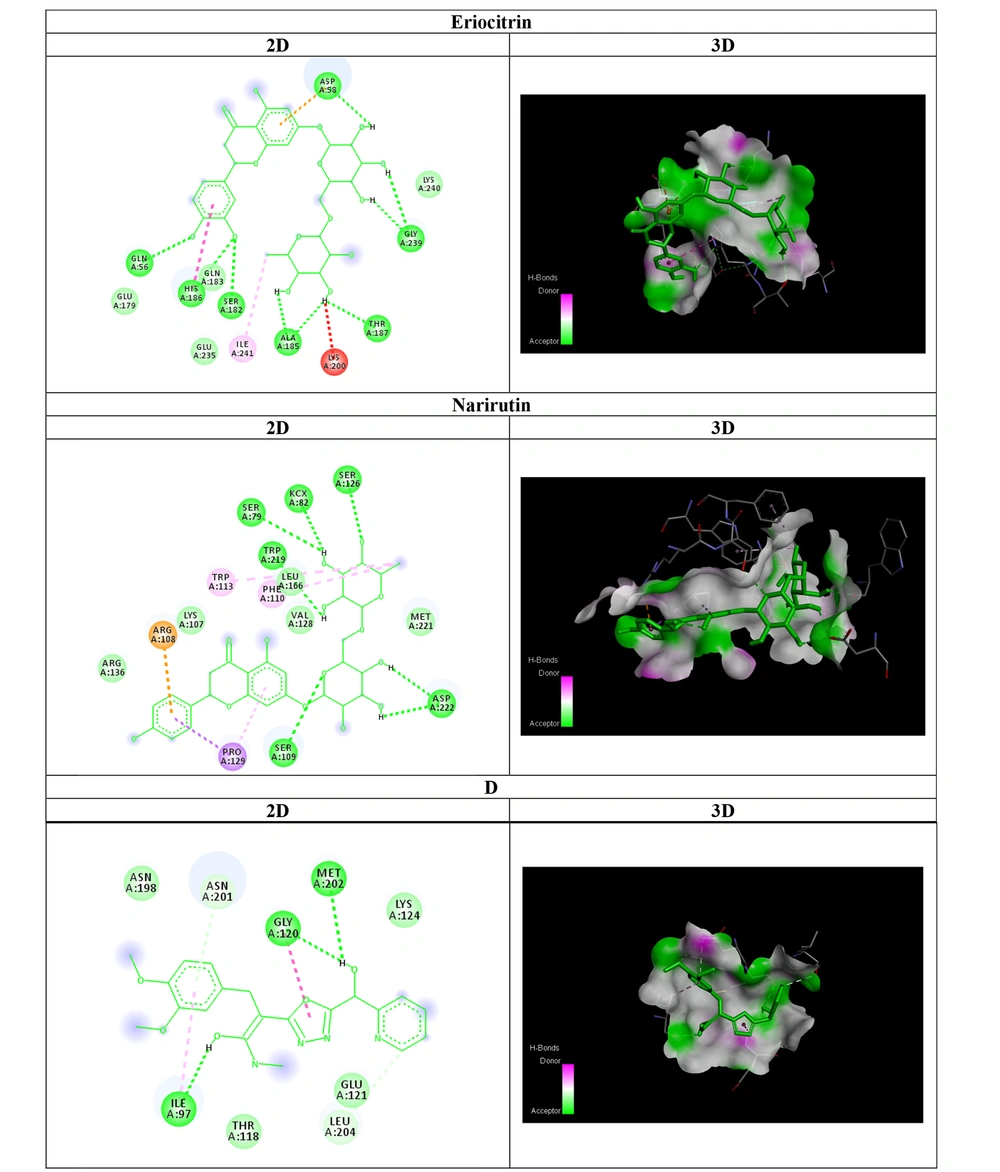
As shown in Table 2, all affinities of all compounds against OXA-23 were calculated. The affinity of flavonoid compounds was reported between - 6.2 and - 9.6. Also, the affinity of oxadiazole derivatives was reported between - 6.7 and - 8.7. Among flavonoid compounds, compounds Eriocitrin and Narirutin showed the highest amount of H-bonds, also these two compounds created the highest amount of variety and number of hydrogen bonds. Among oxadiazole derivatives, compound D showed the highest amount of H-bonds and created the highest amount of variety and number of hydrogen bonds. As shown in Figure 2, Compound Eriocitrin, was involved in hydrogen bonding with an affinity of - 9.4 with the amino acids Aspartic acid: 58, Glycine: 239, Threonine: 187, Alanine:185, Serine: 182, Histidine:186 and Glutamine:56. Compound Narirutin, was involved in hydrogen bonding with an affinity of - 9.6 with the amino acids Serine:79, Lysine (carboxymethylated): 82, Serine: 126, Tryptophan: 219, Aspartic acid:222 and Serine: 109. Also, Compound D ((1E,2E) -3- (3,4-dimethoxyphenyl) -2- (5 -((S) -hydroxy(pyridin -2-yl) methyl) -1,3,4-oxadiazol -2-yl)-N-methylacrylimidic acid), was involved in hydrogen bonding with an affinity of -8.7 with the amino acids Methionine: 202, Glycine: 120 and Isoleucine: 97.
| Compounds(Kcal/mol) | 4k0x | H-Bonds | ||
|---|---|---|---|---|
| Affinity | D.F.R | B.M.R | ||
| Acacetin | - 8.3 | 2.068 | 2.249 | TRP:219/ THR:217/ ARG:259/ SER:79/ SER:109 |
| Caffeic acid | - 6.2 | 1.818 | 5.471 | SER:126/ SER:79/ TRP:219 |
| Chrysin | - 8.3 | 21.167 | 21.881 | LEU:140/ VAL:161/ GLN:159/ GLN:160 |
| Eriocitrin | - 9.4 | 25.795 | 29.797 | ASP:58/ GLY:239/ THR:187/ ALA:185/ SER:182/ HIS:186/ GLN:56 |
| Galangin | - 8.6 | 3.816 | 8.058 | LEU:166/ ASP:222 |
| Kaempferol | - 8.5 | 0.0 | 0.0 | THR:217/ ARG:259/ SER:79/ TRP:219 |
| Myricetin | - 8.5 | 1.257 | 6.708 | TRP:113/ TRP:219/ THR:217/ ARG:259 |
| Narirutin | - 9.6 | 6.223 | 12.263 | SER:79/ KCX:82/ SER:126/ TRP:219/ ASP:222/ SER:109 |
| Pinobanksin | - 9.0 | 21.171 | 22.189 | ARG:136/ LEU:140/ VAL:161/ GLN:159/ GLN:160 |
| Quercetin | - 8.5 | 4.199 | 7.280 | SER:109/ SER:126/ KCX:82/ SER:79/ GLN:132 |
| A | - 8.3 | 4.191 | 6.681 | SER:79/ TRP:219 |
| B | -6.7 | 0.0 | 0.0 | - |
| C | - 7.1 | 24.803 | 26.156 | THR:57/ GLN:56/ SER:182 |
| D | - 8.7 | 17.244 | 19.524 | MET:202/ GLY:120/ ILE:97 |
Abbreviations: D.F.R, distance from RMSD; B.M.R, best mode RMSD; TRP, tryptophan; THR, threonine, ARG, arginine, SER, serine, LEU, leucine, VAL, valine, GLN, glutamine; ASP, aspartic acid; GLY, glycine; ALA, alanine, HIS, histidine; MET, methionine; ILE, isoleucine).
5. Discussion
Acinetobacter baumanniihas become a significant threat in healthcare settings worldwide, particularly due to its ability to develop resistance to multiple antibiotics. It is often associated with nosocomial (hospital-acquired) infections, and strains with extensive drug resistance, including carbapenem resistance, have emerged
Finding new substances that can effectively combat A. baumannii is crucial in the face of antibiotic resistance. The treatment options for A. baumannii infections are often limited due to its resistance to many commonly used antibiotics. Infections caused by multidrug-resistant strains of A. baumannii can be challenging to treat, leading to increased morbidity, mortality, and healthcare costs. Studying and identifying substances with activity against A. baumannii can provide valuable insights into the mechanism of action of these substances and the vulnerabilities of the bacterium (24). According to this, Nocera et al., emphasized it is very important to find new compounds showing antimicrobial and antibiofilm properties against A. baumannii (25). Eriocitrin is a flavonoid compound found in citrus fruits, particularly in the peels. While there is limited specific research available on the antibacterial effects of eriocitrin, flavonoids, in general, have been studied for their potential antimicrobial properties. Flavonoids exhibit a wide range of biological activities, including antibacterial effects against various bacterial strains. It’s important to note that the antibacterial activity of eriocitrin and other flavonoids can vary depending on the specific bacterial strain being tested, the concentration and purity of the compound used, and the experimental conditions. While eriocitrin specifically has limited available data on its antibacterial effects, it is plausible that it may possess similar properties to other flavonoids. However, further research is necessary to specifically evaluate the antibacterial effects of eriocitrin against different bacterial strains, including A. baumannii (26). Narirutin is a flavonoid compound primarily found in citrus fruits, such as grapefruits and oranges. While research specifically focusing on the antibacterial effects of narirutin is limited, flavonoids, including narirutin, have been investigated for their potential antimicrobial properties. While narirutin specifically has limited dedicated research on its antibacterial effects, it is reasonable to speculate that narirutin, as a flavonoid, may possess similar properties to other flavonoids (27). According to this, Abirami et al., reviewed the antimicrobial effects of herbal compounds, they reported that Makrut leaf oil (citronellal) and makrut oil (limonene, terpinene and a- terpineol) exhibited antibacterial properties by disc diffusion method against M. catarrhalis, H. influenza,S. pneumonia, S. aureus and A. baumannii(28). The anti-A. baumannii effects of oxadiazole compounds containing a methoxyphenyl group can vary depending on the specific compound and its structural characteristics. Oxadiazoles are a class of heterocyclic compounds that have been investigated for their antimicrobial properties, including their potential activity against A. baumannii. Research studies have explored the antimicrobial effects of various oxadiazole derivatives against A. baumannii. Oxadiazole derivatives have demonstrated broad-spectrum antimicrobial activity against a range of bacterial strains, including A. baumannii. The activity can vary depending on the specific substituents and structural features of the oxadiazole compound. The antimicrobial effects of oxadiazole compounds are believed to involve multiple mechanisms. They can disrupt bacterial cell membranes, inhibit essential enzymes or proteins, interfere with DNA replication, and induce oxidative stress, leading to bacterial cell death or growth inhibition. The antimicrobial activity of oxadiazole compounds is influenced by their chemical structure. Substituents on the oxadiazole ring, including the methoxyphenyl group, can impact the activity and selectivity against A. baumannii (29). According to this, Christoff et al, Synthesis of novel 1,2,5-oxadiazoles and evaluation of action against A. baumannii, they reported that derivative (E) -3-(2-hydroxyphenyl) -N- (4-methyl-1,2,5-oxadiazol-3-yl) acrylamide can inhibitor of A. baumannii (30). In this research, derivative B ((1E,2E) -3-(3,4-dibromophenyl) -2-(5-((S) -hydroxy(pyridin-2-yl)methyl) -1,3,4- oxadiazol-2-yl) -N- methylacrylimidic acid) and D ((1E,2E) -3- (3,4-dimethoxyphenyl) -2- (5-((S)- hydroxy(pyridin-2- yl)methyl) -1,3,4-oxadiazol-2-yl) -N methylacrylimidic acid) showed strong antibacterial activity against A. baumannii. It is possible that the synthesized structures containing dibromophenyl and dimethoxyphenyl functional groups cause antibacterial effect. In this regard, Tresse et al. synthesized and evaluation of 1,3,4-oxadiazole derivatives for development as broad-spectrum antibiotics, their results showed the three new oxadiazole compounds CT1-69, CT1-83, and CT1-115 have antimicrobial activities that are higher than the KKL-35 reference molecule against a large panel of gram-positive pathogenic strains (18). On the other hand, In silico results showed that the ability of flavonoid compounds especially Eriocitrin and Narirutin to inhibit OXA-23 was higher than others due to its high and strong hydrogen bonds. Also, Compounds A-D were evaluated as a ligand to inhibit the OXA-23 receptors. The results showed that the ability of compound D to inhibit OXA-23 was higher than others due to its high and strong hydrogen bonds. In this regard, Kapoor and Bharadvaja study Virtual Screening and Molecular Docking Analysis of Phytochemicals Derived from Woodfordia Fruticosa To Delineate A. baumannii OXA-23 Antagonists, the reported that Maslinic acid and Oleanolic acid were the top lead molecules with binding energies as high as 9.4 kcal/mol and - 9.0 kcal/mol respectively (31).