1. Background
Paranoid personality disorder (PPD) is a personality disorder characterized by certain symptoms such as unfounded mistrust and suspicion of others. Individuals with this disorder are consistently cautious and skeptical of other people, believing that others are constantly attempting to belittle, harm, or threaten them (1). These baseless beliefs and habits of blaming and mistrusting others can hinder their ability to establish close relationships (2). Paranoid personality disorder typically begins in early adulthood and seems more prevalent in men than women (3, 4). Research suggests that the exact etiology of PPD is unknown, although it is likely that its cause involves a combination of biological (genetic) and psychological factors (5, 6). The prevalence of PPD in individuals with close relatives who have schizophrenia indicates a potential genetic link between the two disorders. However, early childhood experiences, e.g., physical or emotional trauma, can also contribute to the development of PPD (7).
Impulsivity is a major symptom of PPD. Patients with PPD exhibit impulsivity in at least two potentially harmful areas (8). In other words, they are prone to overeating, risky and unpredictable sexual behavior, substance abuse, careless spending, and reckless driving (9). Impulsivity is characterized by a tendency to quickly and spontaneously react to internal and external stimuli, regardless of the outcomes (10). The behavioral perspective considers impulsivity to involve prioritizing short-term gains, which often have little value, over more valuable long-term gains (11, 12). The Reward Deficiency Syndrome (RDS) theory states that impulsive behaviors, despite potentially damaging to some extent, set up the possibility of receiving a reward (13). Reward Deficiency Syndrome is associated with various types of addictions and behaviors that involve seeking rewards (14-16). Individuals with RDS tend to seek out highly intense emotions (14).
Paranoid personality disorder is linked to the development of anxiety in patients (17). Anxiety emerges as a dull, generalized, and unpleasant sense of apprehension and fear whose origin remains unknown (18, 19). It entails desperation, helplessness, uncertainty, and physiological arousal. Anxiety is caused by the repetition of past stressful situations or circumstances in which a person has been injured (20). Anxiety has the potential to disrupt a person's cognitive abilities, leading to negative cognition (21). Many studies have demonstrated that anxiety can affect cognitive functions and working memory (22, 23). Anxiety can be defined as a response to ambiguous and unclear hazards (24). In other words, it is a sense of uneasiness and annoying fear that arises from anticipating a danger with an unclear origin.
To deal with anxiety in patients with PPD, in most cases, anti-anxiety drugs such as benzodiazepine are used (25). However, the treatment of choice for PPD is psychotherapy. Paranoid personality disorder treatment focuses on enhancing general coping skills, improving social interaction, refining communication, and boosting self-esteem. Treating individuals with PPD can be challenging, for trust plays a key role in psychotherapy, and people with PPD tend to have a general distrust of others (26). As a result, many patients with PPD fail to adhere to their treatment plans. Recently, noninvasive brain stimulation has been employed to treat several psychiatric and neurological disorders.
The transcranial direct-current stimulation (tDCS) technique is growing in popularity as a means of manipulating brain activity (27). In this method, a weak electric current enters the nervous tissue through the skin and the skull, altering the excitability of the brain tissue (28). Typical protocols involve the stimulation of the cranial wall using direct current through two electrodes attached to the skin. One electrode serves as the anode, whereas the other functions as the cathode. An electric current of 1 – 2 milliamps is applied for 20 minutes between these two electrodes, which are 35 cm2. The current direction is from the anode to the cathode. Depending on the direction and intensity of the current, the excitability of the cerebral cortex can either increase or decrease (29). Research studies conducted worldwide have confirmed the positive impact of tDCS on improving impulsivity, reducing rumination and anxiety, and alleviating all forms of anxiety and tension (30-33). At the same time, tDCS has shown significant results as a promising intervention for reducing paranoia in both clinical and normal populations (34, 35). The pursuit of novel and efficient treatments has become a fundamental concern for psychologists and psychiatrists due to the considerable number of paranoid patients and the emergence of different complications associated with this condition. However, no research has analyzed the impact of tDCS on the psychological problems of patients with PPD.
2. Objectives
Based on the issues outlined in the background, the present study aimed to investigate the effects of tDCS on impulsivity and anxiety in patients with PPD. The two hypotheses of the present study were as follows: (1) tDCS is effective in improving impulsivity in patients with PPD; (2) tDCS reduces anxiety in people with PPD.
3. Methods
3.1. Study Design
This quasi-experimental research adopted a pretest-posttest control group design.
3.2. Participants
The statistical population included all patients aged 30 – 50 years with PPD referred to psychological and psychiatric clinics and hospitals in Yazd, (Iran), in 2022. Convenience sampling was used in this study. After a psychiatrist confirmed PPD, 30 individuals were randomly selected and placed in an experimental group (n = 15) and a control group (n = 15) using a table of random numbers. The specified sample size was selected based on GPower software (with an effect size of 1.59, a test power of 0.90, and a significance level of 0.05) (36). The inclusion criteria were as follows: An above-average score on impulsivity and anxiety questionnaires, age between 30 and 50 years, confirmation of PPD presence by a psychiatrist, absence of concurrent participation in other treatment programs, and consent to participate in the research. Inability to attend more than two sessions of the treatment, concurrent use of other therapies (e.g., psychological treatments), comorbid disorders, and lack of motivation to participate in the treatment were considered the exclusion criteria. Once the treatment sessions were finished, the study groups received the posttest measures under similar conditions. Study participants were assured that their participation in the study would remain confidential and only the results would be reported. Informed consent was also obtained from the participants.
3.3. Tools
3.3.1. Barratt Impulsiveness Scale
Barratt (37) developed this 30-item self-report scale. The participant responds to the items on a 4-point Likert Scale ranging from 1 (never/rarely) to 4 (almost always/always). Out of the 30 items on this scale, 11 are scored inversely. The minimum and maximum scores on this scale are 30 and 120, respectively. The Cronbach alpha of the Persian Barratt Impulsiveness Scale was 0.84 (38).
3.3.2. Beck's Anxiety Scale
Designed by Beck, the anxiety scale consists of 21 items that aim to measure the severity of anxiety. It is employed to assess the severity of anxiety in the past week on a scale from "not at all" to "severely." The score for each item ranges from 0 to 3, with a total score range of 0 to 63. A higher score indicates a higher level of anxiety (39). Kaviani and Mousavi (40) reported an alpha Cronbach coefficient of 0.82 for the Beck Anxiety Scale.
3.4. Data Collection
3.4.1. The Transcranial Direct-Current Stimulation Device
The iOMED device, made in the United States, was used for transcranial stimulation with electrical current. It has two separate channels, each of which can be adjusted to apply stimulation. The parameters of time, current intensity, and frequency can be adjusted in this device. The device is equipped with a rechargeable battery and has an anode electrode for excitation and a cathode electrode for inhibition. The electrodes are placed in a saline-soaked pad for electrical conduction. The subjects in the experimental group underwent tDCS for 10 sessions, each lasting for 20 minutes (41).
After receiving the ethical permits, the psychology and psychiatry clinics and hospitals of Yazd City were referred to in order to select the patients with PPD. After selecting the sample, they were given the research objectives and ethical considerations, including data confidentiality. Before starting the intervention program, both experimental and control groups were evaluated with research questionnaires. Participants in the experimental group underwent twelve 20-minute sessions of tDCS treatment. The control group remained on the waiting list at this time. After the end of the intervention sessions, the research variables in both groups were measured again using research tools.
3.5. Data Analysis
The data were analyzed using descriptive statistical measures (mean and standard deviation) and analysis of covariance (ANCOVA). The normal distribution of the data was done using the Shapiro–Wilk test. Paired t-test and chi-square test were used to compare the demographic variables of the intervention and control groups. Data analysis was done with SPSS-25. The level of significance was set at P < 0.05.
4. Results
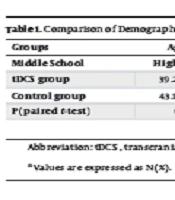
The participants included 30 males and females with PPD. The mean age of the experimental group was 39.27 ± 7.52 years, whereas that of the control group was 43.19 ± 9.27 years old. No significant difference was observed between the experimental and control groups in terms of demographic variables (P > 0.05) (Table 1).
| Groups | Age (y) | Duration of PPD Diagnosis (y) | Education | Gender | |||
|---|---|---|---|---|---|---|---|
| Middle School | High School | College Education | Male | Female | |||
| tDCS group | 39.27 ± 7.52 | 5.33 ± 2.69 | 4 (30.0) | 7 (40.0) | 4 (30.0) | 9 (60.0) | 6 (40.0) |
| Control group | 43.19 ± 9.27 | 6.50 ± 2.91 | 3 (35.0) | 6 (45.0) | 6 (20.0) | 8 (53.3) | 7 (46.7) |
| P (paired t-test) | 0.214 | 0.263 | 0.734 | 0.717 | |||
Abbreviation: tDCS, transcranial direct-current stimulation.
a Values are expressed as No. (%).
There was a significant difference in the mean scores of the pretest and posttest impulsivity and anxiety in patients with PPD (P < 0.001). However, no significant changes were observed in the control group (P > 0.05) (Table 2).
| Variables and Phases | tDCS Group | Control Group | P (Between Group) |
|---|---|---|---|
| Impulsivity | |||
| Pretest | 93.80 ± 3.48 | 94.80 ± 4.63 | 0.509 |
| Posttest | 54.67 ± 4.03 | 93.93 ± 3.67 | 0.001 |
| P (within group) | 0.001 | 0.573 | - |
| Anxiety | |||
| Pretest | 42.40 ± 2.32 | 42.33 ± 2.96 | 0.943 |
| Posttest | 16.40 ± 2.79 | 42.39 ± 2.99 | 0.001 |
| P (within group) | 0.001 | 0.956 | - |
Abbreviation: tDCS, transcranial direct-current stimulation.
a Values are expressed as mean ± SD.
The ANCOVA was employed to assess the significance of differences between the two groups. The results of the Shapiro-Wilk test for impulsivity in the pretest (W = 0.95, P = 0.211) and posttest (W = 0.91, P = 0.118) stages, as well as the anxiety variable in the pr-test (W = 0.89, P = 0.115) and pretest (W = 0.88, P = 0.328) stages, showed the normal distribution of the data. The normality of the data distribution was established to perform the ANCOVA.
To assess the impact of tDCS intervention on impulsivity and anxiety in patients with PPT, a univariate covariance analysis was conducted. Table 3 reports the results from the posttest stage. In Table 3, the calculated effect sizes of impulsivity and anxiety indicate that the independent variable (tDCS) accounted for 96% and 92% of the total variances in these traits for the experimental group and the control group, respectively. The statistical power of the test was equal to 1, indicating the adequacy of the sample size. Therefore, tDCS was effective in improving impulsivity and reducing anxiety in patients with PPT (P < 0.001) (Table 3).
| Dependent Variables | SS | df | MS | F | P | η2 | Power |
|---|---|---|---|---|---|---|---|
| Impulsivity | 11 440.85 | 1 | 11 440.85 | 811.00 | 0.001 | 0.96 | 1.00 |
| Anxiety | 4 929.44 | 1 | 4 929.44 | 560.81 | 0.001 | 0.92 | 1.00 |
5. Discussion
This study aimed to determine the effectiveness of tDCS on impulsivity and anxiety in patients with PPD. The finding indicated that tDCS effectively reduced impulsivity in patients with PPD. This finding is consistent with the results reported by other studies that have demonstrated the impact of brain tDCS on improved impulsivity (42, 43). Impulsivity is characterized by an abrupt and unwelcome response to a stimulus before a comprehensive assessment of the information is conducted. Today, impulsivity is conceptualized as a neurobiological dimension. In other words, impulsivity is linked to cognitive disinhibition, issues with neurotransmitters, and emotional instability (40). Patients' high impulsivity may explain their difficulties in delaying needs and inhibiting behaviors. Therefore, patients' impulsive actions, movements, and behaviors may be attributed to their inability to restrain, control, and manage their impulses. A major characteristic of tDCS is its ability to create cortical changes after the stimulation has ended (43). The electrical stimulation of the brain is a treatment method that relies on the neuroplasticity of the central nervous system to treat a range of psychiatric and neurological disorders.
It can be inferred that tDCS alters neuron excitability and shifts the membrane potential of surface neurons toward depolarization or hyperpolarization. This, in turn, leads to an increase or decrease in the firing of brain cells. Most likely, anode stimulation causes an increase in brain excitability and normalization of nervous system functioning. Transcranial direct anodic stimulation in the left dorsolateral prefrontal region decreases and alters the effectiveness of the brain region responsible for impulsive behaviors. In general, tDCS is a neuropsychological test used to treat emotional disorders, particularly those stemming from neurodevelopmental causes. It is used to either stimulate or inhibit cognitive and motor abilities. Moreover, tDCS is a noninvasive method for stimulating neuronal function in the brain. It relies on the magnetic field's capacity to penetrate the skull and meninges, thereby inducing an electrical current in the brain tissue. The electrical current that reaches this area causes the neurons to carry an electric charge, creating positive and negative polarity. This, in turn, alters the activity of that particular area. Furthermore, tDCS enhances excitability in specific areas of the brain, which has been associated with changes in cognitive and behavioral performance and reduced impulsivity in people (42).
The results also demonstrated that the tDCS was conducive to reducing anxiety in patients with PPD. This finding is consistent with the research results of previous studies (30, 31). In the tDCS method, the anodic current is used to increase cerebral cortex excitability, and the cathodic current is used to decrease it. In this method, electrodes are placed on the patient's head to pass a continuous and mild electric current through it. Essentially, tDCS enhances excitability in the targeted areas of the brain, leading to cognitive and behavioral performance changes in individuals. The current is supplied by a direct current generator powered by a battery. Through this current, long-term changes occur in the polarity of the cerebral cortex. These changes result from the depolarization and hyperpolarization of neurons and the impact on nerve receptors (30). Overall, tDCS is a neurological treatment technique that involves the introduction of a direct and weak current to cortical areas. This current is aimed at facilitating spontaneous neural activities. By stimulating the cortex of specific areas, tDCS can potentially improve or reduce brain functions.
The research had certain limitations. Since this study was conducted on patients with PPD in the city of Yazd, it is important to exercise caution while generalizing the results to patients in other cities. Among the other limitations of the current research, we can mention the convenience sampling and the small sample size.
5.1. Conclusions
According to the research findings, we can conclude that tDCS effectively reduced anxiety and impulsivity in patients with PPD. Therefore, it is recommended that psychiatrists, psychotherapists, and psychological service centers employ this treatment method as a complementary approach to address the psychological problems of patients with PPD. In conclusion, it should be noted that tDCS may be used as a complementary method for treating paranoid anxiety; however, it should not be relied on as the primary treatment method.