1. Background
The liver plays a crucial role in maintaining overall health by filtering toxins, producing essential nutrients, and regulating blood clot formation (1). However, the liver is susceptible to damage from hepatotoxins (2, 3). Prolonged exposure to toxic substances can lead to severe and irreversible liver damage, as the liver’s remarkable regenerative capacity becomes overwhelmed, ultimately resulting in significant morbidity and mortality (4). Carbon tetrachloride (CT) is a toxic organic compound that has been associated with severe liver damage in humans, primarily through its ability to induce oxidative stress, which triggers lipid peroxidation in liver parenchymal cells. This oxidative stress-mediated damage can lead to significant hepatic dysfunction (5).
Traditional medicine plays an important role in healthcare systems worldwide (6-9). Fumaria Parviflora Lam is an annual herb that has been traditionally used to treat various disorders, including hepato-biliary dysfunction (10). The phytochemicals derived from this plant are used as a potent bitter tonic, astringent, laxative, diuretic, and alternative therapeutic agent, showcasing its multifaceted medicinal properties and potential applications in complementary and integrative medicine (11). Fumaria Parviflora L. contains a diverse array of bioactive compounds, including flavonoids, glycosides, tannins, saponins, steroids, triterpenoids, phenols, and alkaloids, which contribute to its complex pharmacological profile and therapeutic applications (10). Fumaria Parviflora L. has been extensively studied and found to exhibit a wide range of pharmacological activities, including anti-inflammatory, antispasmodic, antidiarrheal, bronchodilator, hypoglycemic, anthelmintic, laxative, antiprotozoal, dermatological, and hepatoprotective effects, underscoring its potential as a valuable therapeutic agent (12).
2. Objectives
Based on these considerations, this study was designed to assess the potential protective effects of FP on hepatotoxicity induced by CT in HepG2 cells.
3. Methods
3.1. Fumaria Parviflora L. Hydroalcoholic Extract Preparation
The FP extract was prepared through five distinct stages: Plant collection from a university-associated medical plant center, plant identification (conducted by an expert botanist or taxonomist), plant drying and grinding (the plant was dried at room temperature in a well-ventilated area away from direct sunlight, followed by grinding), FP extraction (a mixture of 1 L of 80% ethanol (Merck, Germany) and 100 g of FP was combined), and incubation of the solution for 7 days. Finally, the extract was filtered to remove plant material and insoluble components, then dried. The resulting extract was refrigerated for later use (13).
3.2. HepG2 Cell Culture
The HepG2 cell line (an immortalized cell line derived from human liver carcinoma, commonly used as an in vitro model for studying various aspects of liver biology and pathology) was cultured in Eagle's minimum essential medium (EMEM) (Gibco, Germany), supplemented with 10% fetal bovine serum (FBS) (Gibco, Germany) (14).
3.3. Cell Viability Assay
The cells (15,000 per well) were cultured in 96-well plates. After 24 hours, concentrations of 3.12, 6.25, 12.5, 25, 50, 100, and 200 μg/mL of FP or 0.15, 0.31, 0.62, 1.25, 2.5, 5, and 10 µM of CT were added to the wells. Following a 24-hour incubation period, 40 μL of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Gibco, Germany) solution was added to each well. After 4 hours, the MTT solution was removed, and 100 μL of DMSO was added to each well, followed by incubation for 2 hours in the dark at room temperature. The level of light absorption was measured using an enzyme-linked immunosorbent assay (ELISA) reader at a wavelength of 570 nanometers. Subsequently, the percentage of viable cells was calculated using the following formula (15):
The IC50 for CT was calculated using GraphPad Software (v5, Inc., San Diego, USA). The cells were then cultured in 96-well plates and treated with non-toxic concentrations of FP and the IC50 value of CT. Three distinct experimental designs were employed: Pre-treatment, simultaneous treatment, and post-treatment.
In the pre-treatment study, the cells were initially incubated with varying concentrations of FP for 24 hours. Afterward, the medium containing the FP extract was removed and replaced with fresh medium supplemented with the IC50 concentration of CT, followed by an additional 24-hour incubation. In the simultaneous treatment, the cells were treated with FP and CT at the same time and incubated for 24 hours. In the post-treatment study, the cells were treated with the IC50 concentration of CT for 24 hours. Then, the culture medium was replaced with fresh medium containing the FP extract, and the cells were incubated for an additional 24 hours. Cell viability was evaluated using the MTT assay (16).
3.4. Toxicity Assay
The cells (70,000 per well) were seeded in 24-well plates. Subsequently, 500 μL of culture medium containing varying concentrations of the test substance was added to the wells and incubated for 24 hours. Following the incubation period, 100 μL of the culture medium was transferred, and lactate dehydrogenase (LDH) enzyme activity was measured using a cytotoxicity detection kit (Roche Chemical Co., Germany). The absorbance was recorded at 490 nanometers for each sample (17).
3.5. Cell Apoptosis Investigation
Following cell treatment, the percentage of DNA fragmentation was determined using the diphenylamine assay as described by Cohen and Duke. The percentage of DNA fragmentation was then calculated using the following formula (18):
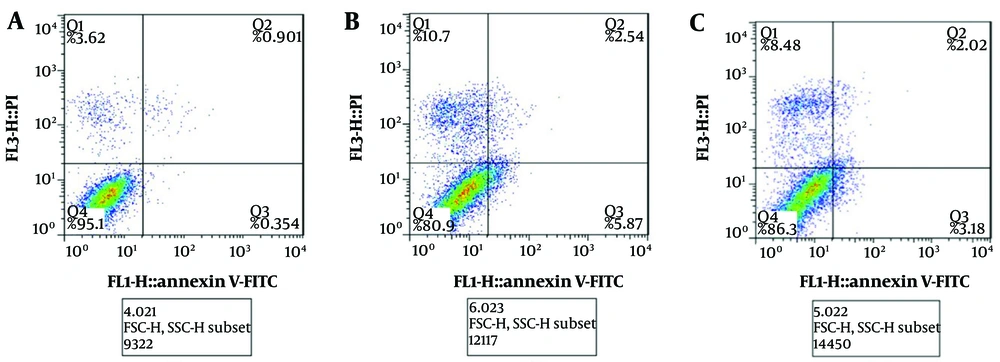
Quantitative estimation of cell death was performed using Annexin V-FITC staining. Briefly, after treatment, the cells were harvested, washed twice with PBS, and suspended in 400 μL of 1× Annexin V binding solution (Sigma, Germany). Five microliters of Annexin V-FITC solution was added to the cell suspension, and the cells were mixed and incubated on ice for 15 minutes in the dark. Then, 10 μL of propidium iodide (PI) staining solution (Sigma, Germany) was added, mixed, and incubated on ice for 5 minutes in the dark. The cells were then analyzed using a flow cytometer.
Additionally, the expression levels of BAX (a pro-apoptotic regulator) and BCL-2 (an anti-apoptotic regulator) were estimated using real-time PCR. After treatment, total RNA was extracted using Thermo Fisher Scientific TRIzol reagent (Waltham, MA, USA) and verified using a nanodrop spectrophotometer and electrophoresis methods. Complementary DNA (cDNA) was synthesized using a Vivantis Technologies kit (Selangor DE, Malaysia), following the manufacturer's protocol. Real-time PCR was conducted using Takara Bio Inc. SYBR Premix Ex Taq Technology (Otsu, Shiga, Japan). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as an internal control. The fold change in the relative expression of each target mRNA was calculated using the comparative Ct (2-ΔΔCt) method. The sequences of the primers were as follows:
BAX (F): 5´-CCTGTGCACCAAGGTGCCGGAACT-3´ and (R): 5´-CCACCCTGGTCTTGGATCCAGCCC-3´; BCL-2 (F): 5´-TTGTGGCCTTCTTTGAGTTCGGTG-3´ and (R): 5´-GGTGCCGGTTCAGGTACTCAGTCA-3´; GAPDH (F): 5´-TCCCTGAGCTGAACGGGAAG-3´and (R): 5´-GGAGGAGTGGGTGTCGCTGT-3´.
3.6. Data Analysis
The t-test was used for data analysis, with results presented as mean ± SD. SPSS software (v.16) was utilized for all statistical analyses, and the significance level was set at P ≤ 0.05 (1).
4. Results
4.1. Effect of Fumaria Parviflora L. Extract on Cell Viability
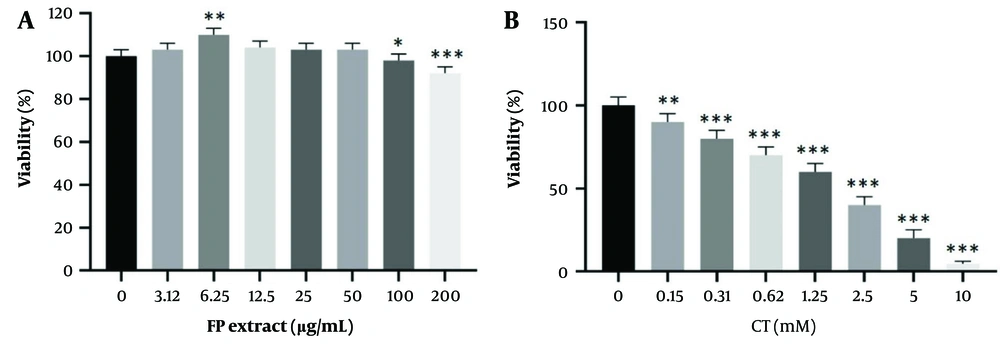
The effects of different FP concentrations on cell viability after 24 hours, as measured by the MTT assay, are shown in Figure 1. A significant decrease (P < 0.05) in cell viability was observed at concentrations of 100 and 200 μg/mL. Additionally, a significant increase (P < 0.05) in cell viability was found at 6.25 μg/mL.
The effect of Fumaria Parviflora (FP) extract (A); and CT (B) on viability rate in HepG2 cells. The survival rate of the cells with different concentrations was evaluated by MTT (after 24 hrs of treatment). Control group received the same volume of medium with no extract. * indicated P < 0.05, ** indicated P < 0.01 and *** indicated P < 0.001 compared to the control cells.
4.2. Effect of CT on Cell Viability
As shown in Figure 1, CT significantly decreased cell survival in a concentration-dependent manner. The IC50 was determined to be 1.05 μM after 24 hours.
4.3. Fumaria Parviflora L. Toxicity on HepG2 Cell
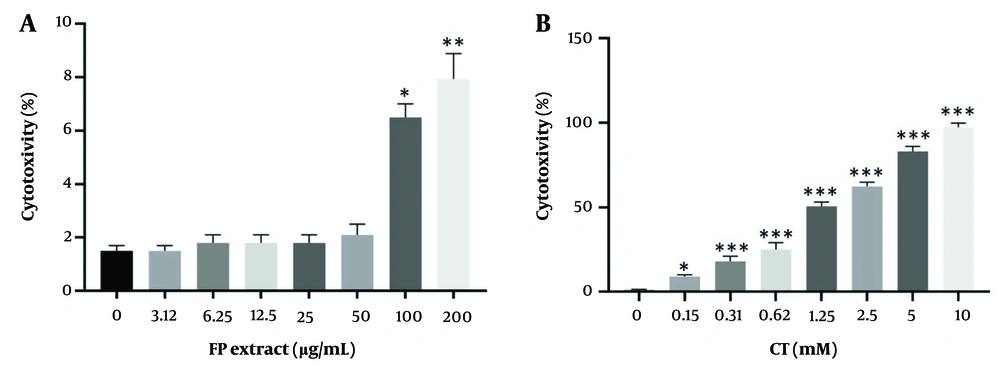
This value was assessed using the lactate dehydrogenase test over 24 hours. The toxicity of the FP extract was significant at concentrations of 100 and 200 μg/mL (P < 0.05) (Figure 2).
The toxicity of Fumaria Parviflora (FP) extract (A); and CT (B) on HepG2 cells. Toxicity value was evaluated by lactate dehydrogenase test after 24hrs of treatment with different concentrations. Control group received the same volume of medium with no extract. * indicated P < 0.05, ** indicated P < 0.01 and *** indicated P < 0.001 compared to the control.
4.4. Carbon Tetrachloride Toxicity on HepG2 Cell
The toxicity effects of different CT concentrations on cell viability after 24 hours, as measured by the lactate dehydrogenase test, are shown in Figure 2. The toxicity rate was found to be significant in a concentration-dependent manner (P < 0.05).
4.5. Simultaneous Effects of Fumaria Parviflora L. Extract and Carbon Tetrachloride on Cell Viability
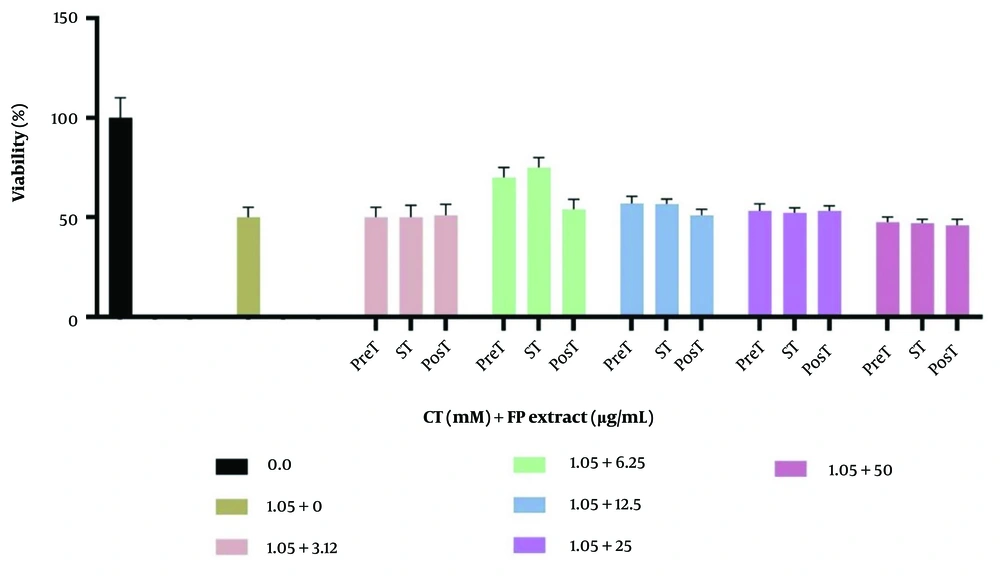
The effects of different concentrations of FP and the IC50 of CT following the three treatment procedures on cell viability are shown in Figure 3. Fumaria Parviflora L. at a dose of 6.25 μg/mL significantly reduced CT toxicity in both the treatment and pre-treatment groups (P < 0.05).
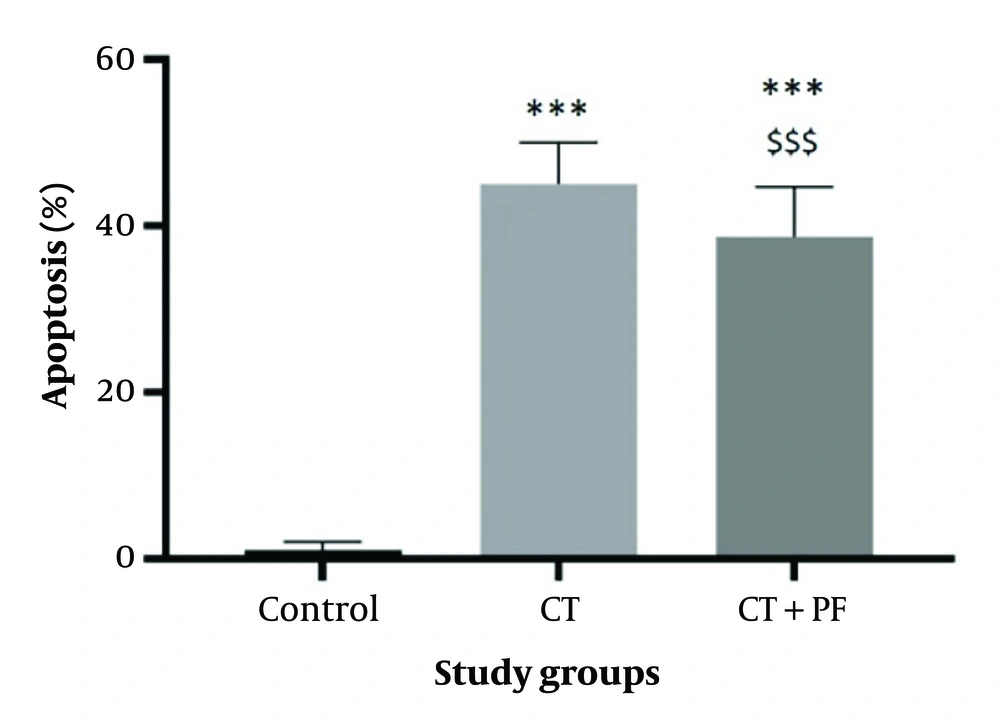
4.6. Effects of Carbon Tetrachloride and Fumaria Parviflora L. Extract on Cell Apoptosis
According to Figure 4, the cell apoptosis rate was significantly increased (P < 0.05) in the CT group. Additionally, the FP extract induced a significant decrease (P < 0.05) in the apoptosis level caused by CT. These findings were further confirmed by flow cytometry analysis (Figure 5).
The effect of CT and/or Fumaria Parviflora (FP) extract on induction of apoptosis in HepG2 cells after 24hrs of treatment assessed by diphenylamine test. Control group received the same volume of medium with no FP. *** indicated P < 0.001 compared to the control and $$$ indicated P < 0.001 compared to the CT group.
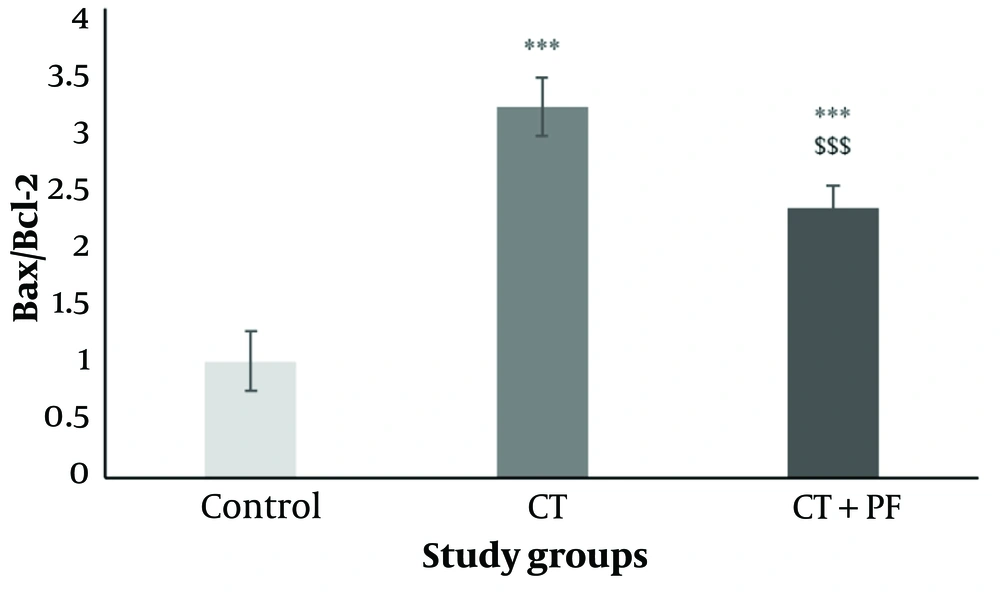
4.7. Effects of Carbon Tetrachloride and Fumaria Parviflora L. Extract on BAX/BCL-2 Expression Ratio
As shown in Figure 6, significant up-regulation of BCL-2 and down-regulation of BAX mRNA expression were observed in the CT group. Consequently, the BAX/BCL-2 ratio was significantly increased by CT treatment (P < 0.05). Additionally, the FP extract induced a significant decrease (P < 0.05) in the BAX/BCL-2 ratio levels elevated by CT.
The effect of CT and/or Fumaria Parviflora (FP) extract on BAX/BCL-2 expression ration in HepG2 cells after 24hrs of treatment assessed by real time PCR test. Control group received the same volume of medium with no FP. *** indicated P < 0.001 compared to the control and $$$ indicated P < 0.001 compared to the CT group.
5. Discussion
In the present in vitro study, the protective effect of the hydroalcoholic extract of FP on CT-induced toxicity in HepG2 cells was investigated through various laboratory assays. According to the findings, the FP extract can stimulate HepG2 cell division at low concentrations, while higher doses of FP can induce toxic effects on these cells. Additionally, CT exhibited concentration-related and time-dependent toxicity on HepG2 cells. In the next stage of the experiment, the protective effects of non-toxic concentrations of FP on CT-induced toxicity were evaluated in three different procedures: Pre-treatment, simultaneous treatment, and post-treatment. The results showed that the most significant protective effect of FP on cytotoxicity occurred during simultaneous treatment.
Carbon Tetrachloride’s hepatic effects are closely related to increased oxidative stress (19-22). Additionally, the study investigated the effects of FP extract on CT-induced apoptosis in HepG2 cells. The results demonstrated that CT exerts its toxic effects by inducing apoptosis, highlighting apoptosis as a potential mechanism of CT cytotoxicity (23). The FP extract exhibited a protective effect by reducing CT-induced apoptosis, likely due to its antioxidant properties. This finding is consistent with previous studies that have demonstrated the antioxidant activity of FP, underscoring its potential role in mitigating the cytotoxic effects of CT (11).
Recent research has highlighted the pivotal role of reactive oxygen species (ROS) and oxidative stress in the induction of apoptosis. Antioxidants have been shown to inhibit or delay the apoptosis process (24, 25). The endogenously produced protein BCL-2 has been found to prevent cell death by apoptosis, likely through antioxidant-mediated mechanisms (26). Accumulating evidence suggests that oxidative stress and apoptosis are closely interrelated physiological processes (27-29). Numerous studies have consistently demonstrated the antioxidant and protective properties of Fumaria Parviflora (FP) in various tissues. For instance, FP extract has been shown to protect testicular tissue and improve sperm quality by mitigating oxidative stress in diabetic rats, highlighting its potential therapeutic applications in the management of testicular dysfunction (30). The ethanol extract of FP leaves has also been found to restore reproductive function suppressed by lead exposure and prevent lead-induced testicular toxicity in male Wistar rats, suggesting its potential efficacy in mitigating the adverse effects of lead on male fertility (31). Additionally, this plant has demonstrated protective effects against testicular tissue changes caused by fluoxetine toxicity (32).
5.1. Conclusions
According to this in vitro investigation, FP demonstrates detoxification properties against hepatotoxicity induced by CT. For future studies, it is recommended to extend this research to animal models of hepatotoxicity.