1. Background
Approximately 85% of stroke survivors experience acute hand dysfunction (1-3). Rehabilitation programs are crucial for improving motor function in individuals requiring therapy. These programs typically involve a prolonged course of treatment delivered by therapists, physicians, or trained family members (1-5).
This research has led to the development and production of a wide range of rehabilitation devices (3-5). A key advantage of such equipment is that it enables active patient participation, facilitating faster recovery by providing patients with greater freedom of movement and reducing the need for repetitive, therapist-guided exercises (6). Traditionally, stroke rehabilitation involves repetitive exercises under the guidance of a physiotherapist to restore range of motion and strength (7, 8). However, traditional treatment methods face limitations that affect patient outcomes, therapist workload, and overall rehabilitation efficiency (9, 10).
In recent decades, the integration of games and virtual reality (VR) into medical applications has revolutionized various aspects of healthcare, extending far beyond their original entertainment purposes. These technologies have found valuable applications in education, diagnosis, treatment, and rehabilitation (11). In medical education, VR and serious games offer immersive and interactive learning experiences for anatomy, imaging techniques, and specialized training. Therapeutic applications have shown promise in mental health treatment, pain management, and physical rehabilitation (5). Notably, the use of VR and games in rehabilitation has emerged as a particularly promising area, enhancing traditional physiotherapy processes. For instance, augmented reality games have been developed to motivate pediatric patients and encourage increased physical activity, making rehabilitation exercises more engaging and effective. These technologies provide safe, controlled, and customizable rehabilitation scenarios tailored to individual patient needs, potentially transforming patient care and therapeutic interventions. As research in this field progresses, we can anticipate more sophisticated and tailored applications of VR and games in medicine, offering innovative solutions for various healthcare challenges and improving patient outcomes (11-17).
This study examined the effects of game-based rehabilitation on neurological recovery using electroencephalography (EEG) signals and the Fugl-Meyer Assessment test. The research focused on a typical rehabilitation group that benefited from game-based interventions. Results demonstrated improvements in Fugl-Meyer scores, attributed to a comprehensive rehabilitation strategy combining motion learning, retraining, and repetitive activities. The system integrated observational movement, mental imagery, VR, and repeated practice methods, enhancing motor learning outcomes. These findings align with previous research, supporting the effectiveness of game-based interventions in neurological rehabilitation (18-21).
2. Objectives
The goal of this study is to explain the technology and usage of home stroke telerehabilitation systems. The objective was to assess the efficacy of a play-based shoulder wheel rehabilitation program for treating individuals with distal shoulder and elbow movement abnormalities.
The EEG signals hold the potential to provide valuable insights into brain activity during rehabilitation. By monitoring brain signals, researchers can gain a deeper understanding of motor learning and recovery processes. Integrating EEG with game-based therapy could enable personalized treatment plans and optimize rehabilitation outcomes.
3. Methods
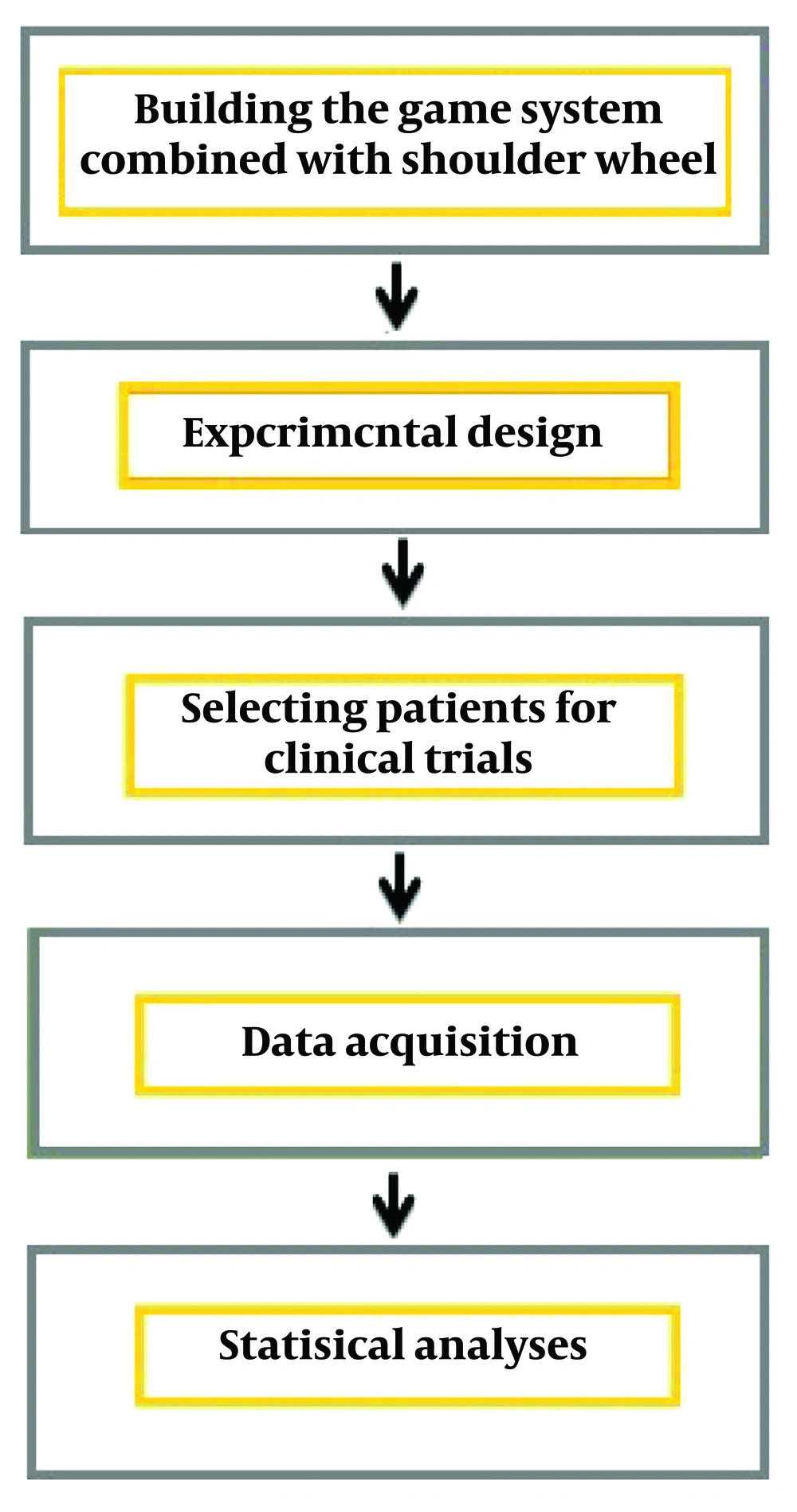
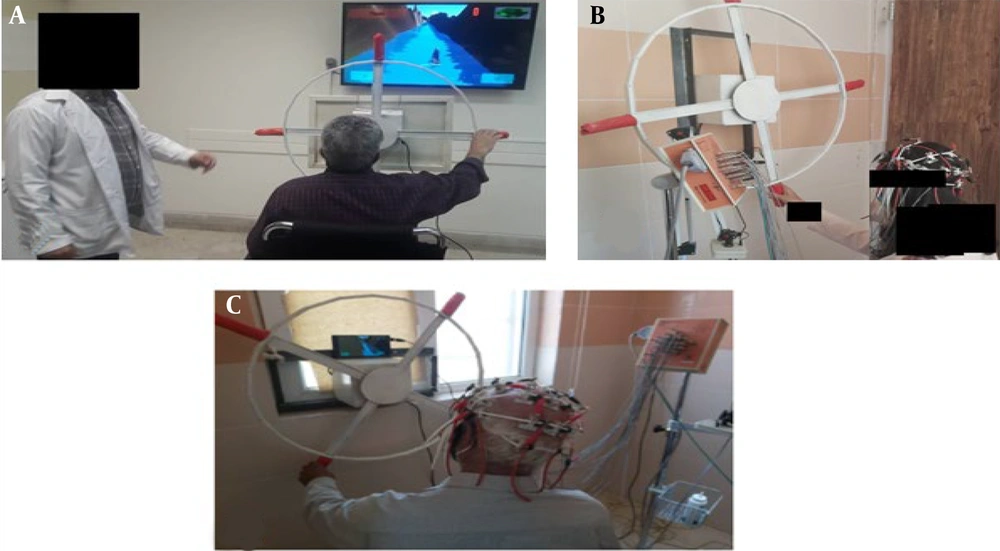
This study developed a game-based telerehabilitation system designed to motivate patients and enhance neuroplasticity by providing visual and auditory feedback tailored to individual conditions. The research methodology is depicted in a block diagram in Figure 1.
3.1. Game System Combined with Shoulder Wheel
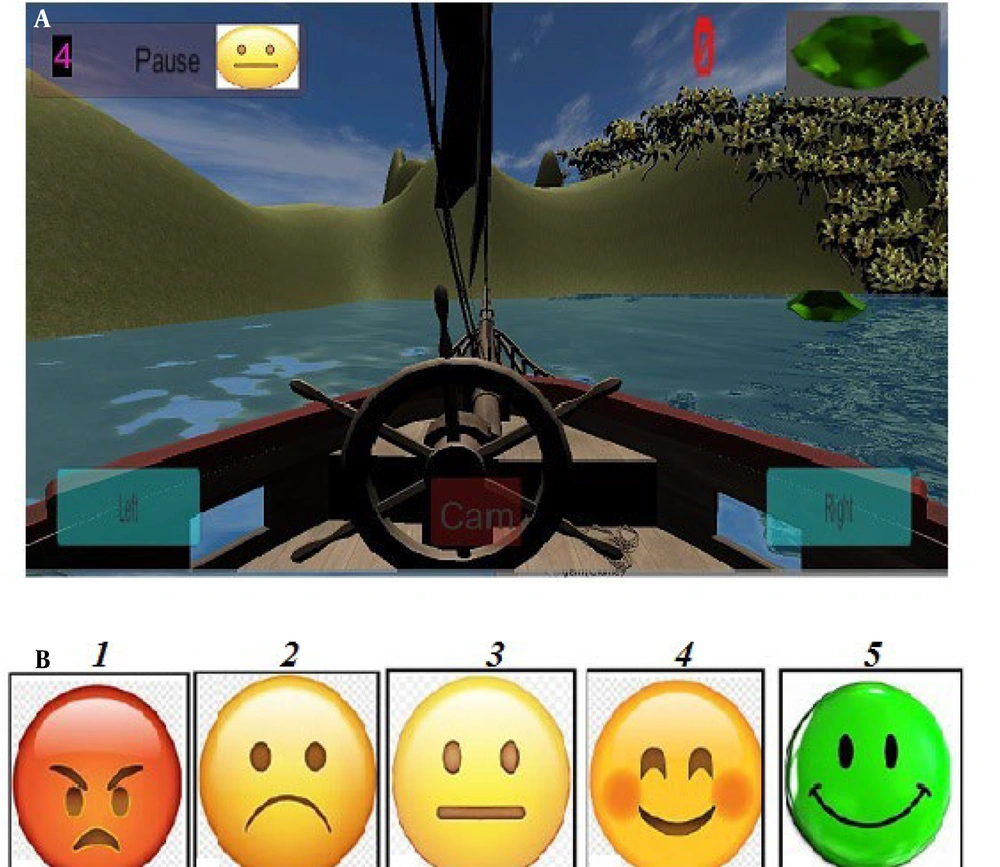
The game-based system was implemented as a phone-based application for home rehabilitation. Figure 2 illustrates the apparatus features utilized in the PC version (22, 23). Both the rehabilitation process and the game being demonstrated can be monitored simultaneously by the therapist.
A game was developed in this study to motivate patients and extend the duration of rehabilitative activities (as shown in Figure 2). The game's path is used for evaluation, while visual graphics provide feedback to the patient (Figure 3).
3.2. Selection of Stroke Patients
The study was a clinical trial approved by the Kermanshah University of Medical Sciences Ethics Committee under the protocol number IR.KUMS.REC.1400.199 and registered with the clinical trial center under code IRCT20210614051570N1. Initially, 120 participants were invited, and 50 were selected, comprising an intervention group of 25 participants (mean age 49 ± 8 years) and a control group of 25 participants (mean age 48 ± 10 years). Stroke patients were recruited from occupational therapy clinics and rehabilitation centers in Hamadan province, meeting the "Vogel-Meier" criterion and passing the hand evaluation exam at least six months post-stroke (24).
Participants were divided into two groups of 25: A control group receiving traditional shoulder and elbow therapy in the clinic and an intervention group treated with a shoulder wheel combined with a remote play-based system for home use. Patients in the control group underwent up to 60 minutes of shoulder wheel exercises without monitor feedback, while the intervention group participated in functional physiotherapy using the remote shoulder and elbow rehabilitation system. The EEG signals were recorded three times during the 45-day rehabilitation phase at 15-day intervals (Figure 4).
3.3. Electroencephalography Signal Recording and Data Processing
An EEG3520 amplifier manufactured by Negar Andishe Argham Company, equipped with 24 monopolar channels, was used for continuous EEG signal recording. Silver-silver chloride electrodes were utilized, following the international 10 - 20 system, with a sampling frequency of 250 Hz. An ear electrode with an impedance of less than 5 kΩ was used as a reference for all channels. During all rehabilitation sessions, brain activity signals were recorded from the following channels: F3-C3, F4-C4, C3-T3, C4-T4, T3-T5, T4-T6, T5-O1, and T6-O2.
Data preparation involved removing noise from the signals and eliminating incomplete data. Noise was filtered out using a zero-phase FIR filter. The PREP and FASTER toolboxes were employed to refine the data by filtering noise channels and retrieving single experimental data from the processed dataset. These toolboxes improved data quality and reliability by effectively removing artifacts and isolating relevant experimental signals, ensuring more precise analysis and interpretation of neurophysiological recordings. To mitigate the effects of blinking and muscle noise, the remaining data were adjusted using the mean of the reference signal and independent component analysis (ICA). The SASICA toolkit was used to further eliminate noise and enhance the overall signal quality.
3.4. Extraction of Rehabilitation Process Evaluation Parameters
The power spectral density (PSD) for each channel was calculated using Welch's periodogram method. The mean absolute PSD values were computed for specific frequency ranges: 1 - 4 Hz (δ), 4 - 7.5 Hz (θ), 7.5 - 12.5 Hz (α), and 12.5 - 30 Hz (β). To evaluate brain asymmetry, two metrics were calculated: The Pairwise-Derived Brain Symmetry Index (pdBSI) and the revised Brain Symmetry Index (rBSI). The pdBSI quantifies asymmetry between pairs of homologous channels in the two hemispheres, while the rBSI provides a measure of overall asymmetry across the brain's hemispheres. These indices provide valuable insights into the spatial distribution of brain activity and highlight potential hemispheric differences in neural processing (25).
4. Results
4.1. Results of Clinical Evaluation of Virtual Reality-Based Rehabilitation System in Stroke Patients
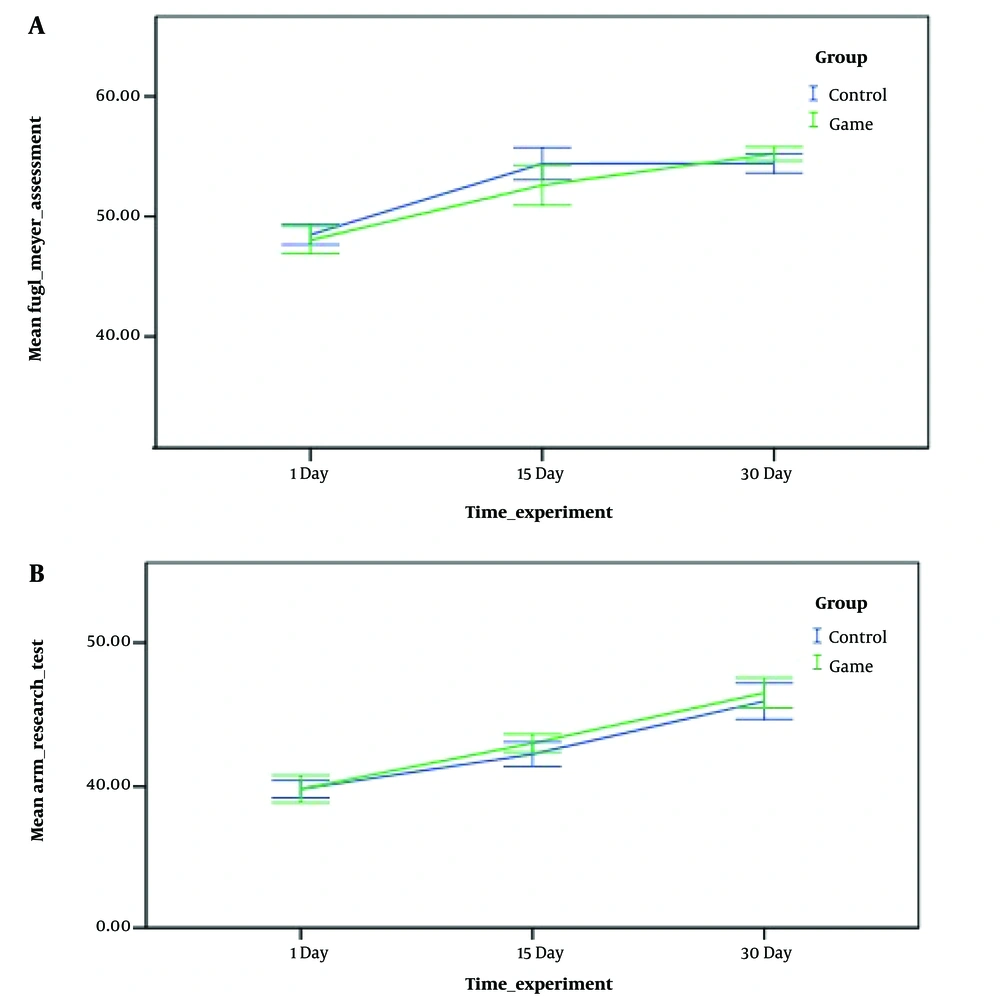
The initial ART and Fugl-Meyer hand evaluation test values were 39 ± 11 and 50 ± 0.5, respectively. Data analysis revealed no statistically significant differences between the two groups at the beginning of the study. The similarity between the groups confirmed that there was no statistically significant difference in the initial evaluation session, indicating the adequacy of the sample randomization (P = 0.362).
At the conclusion of the rehabilitation activities, a comparison of findings revealed that the Fugl-Meyer test results after the exercises in the control and intervention groups were statistically significant (P = 0.033). Moreover, the differences in this parameter between the control and game intervention groups after the rehabilitation activities were significant compared to the prior session of rehabilitation exercises (P = 0.013), as shown in Figure 5.
Additionally, the results from the arm evaluation test following the completion of the rehabilitation activities indicated that the improvements in hand function after training were not statistically significant in either the control or intervention groups (P = 0.073).
4.2. Distribution of Task-Induced Spectral Amplitude Between Cortical Lobes
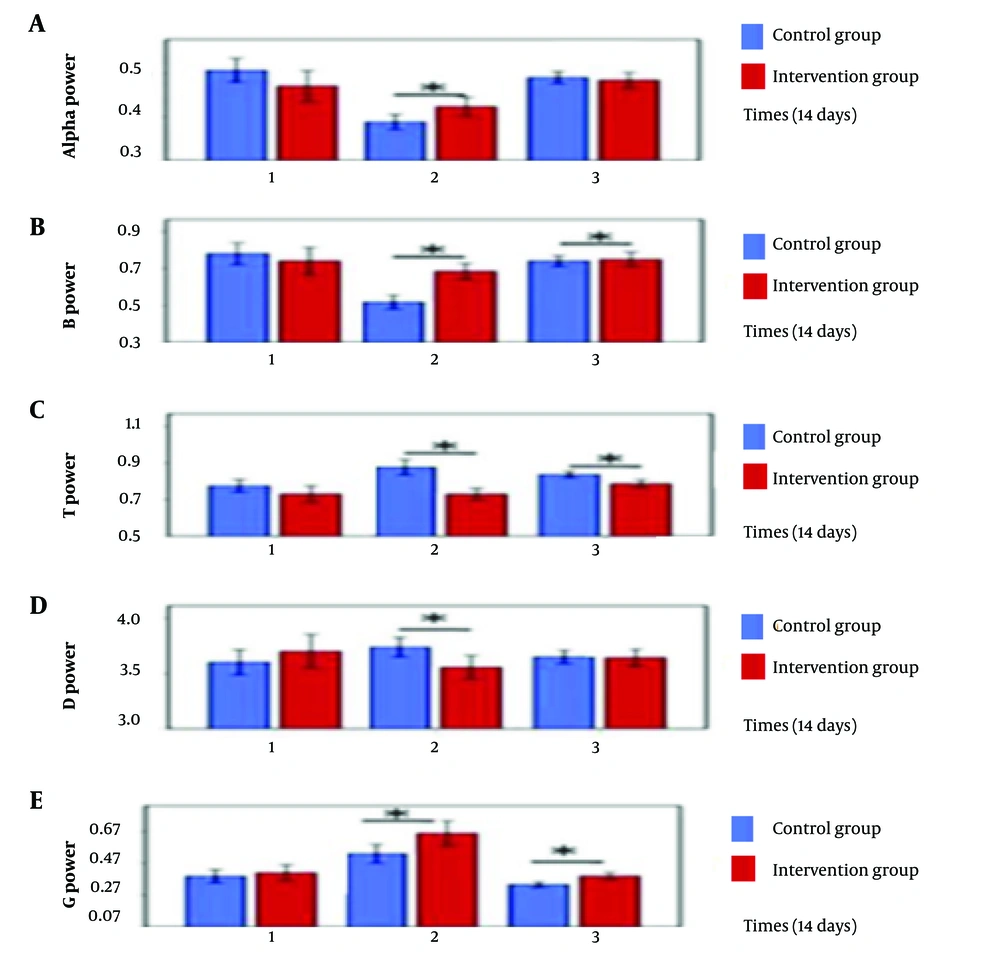
The intervention group demonstrated higher alpha activity in the frontal lobe compared to other lobes at the end of the sessions. Beta waves were predominant in the frontal lobe area in both groups during the third session. The intervention group exhibited stronger theta waves across the entire cortex during the first session, whereas the control group showed powerful theta waves in the occipital lobe during the first and third sessions. Delta waves were strongest in the occipital lobe for the intervention group and were predominant in the occipital, temporal, and central lobes for the control group. Additionally, the intervention group displayed higher gamma wave activity in the frontal brain throughout the sessions. The spectral power of all brain waves (alpha, beta, theta, delta, and gamma) was measured across three 15-day intervals for both groups, as illustrated in Figures 6 and 7.
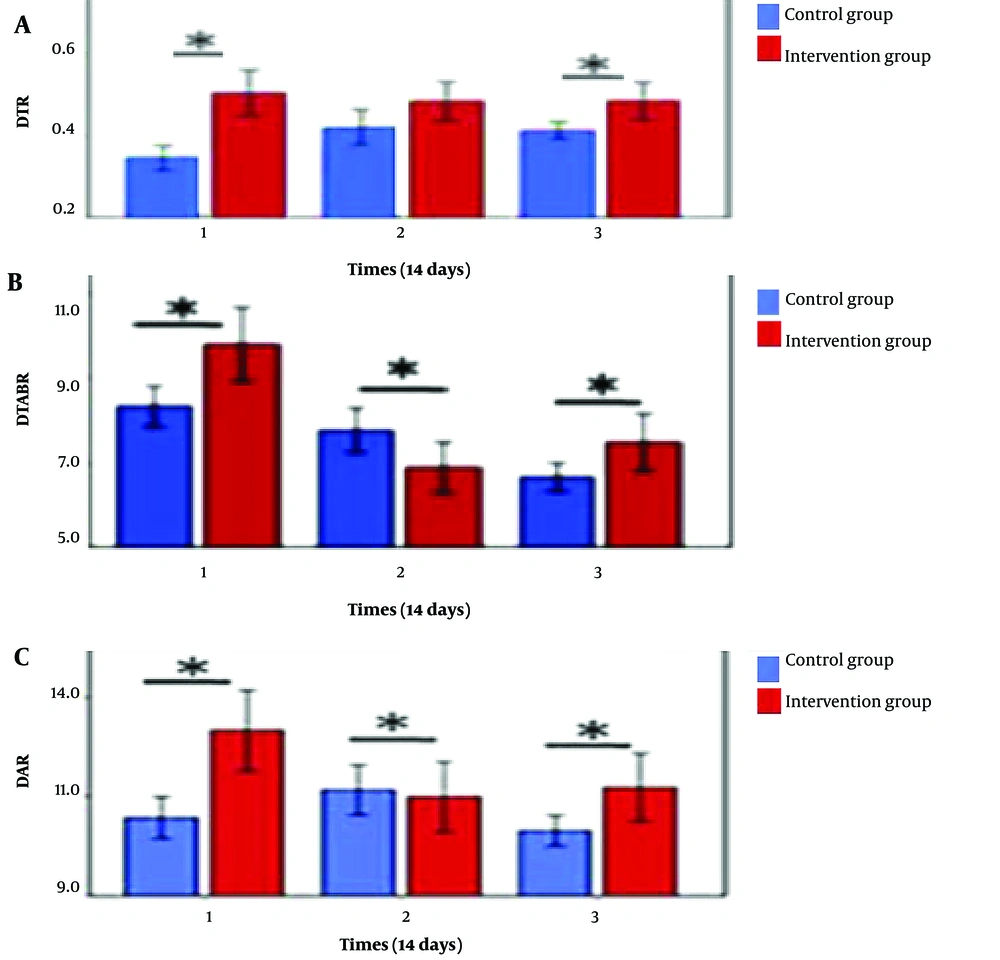
As depicted, DTR, DTABR, and DAR have been suggested as biomarkers for stroke anticipation. During the three sessions, the parameters of DTR, DTABR, and DAR were evaluated to determine their association with stroke-related neurological disease. The outcomes are illustrated in Figure 7.
4.3. Calculation of Correlation Coefficient Between Electroencephalography Signal Parameters and Clinical Parameter of FMA and ART
The correlation between EEG characteristics and clinical parameters of FMA before and after treatment for the intervention and control groups was calculated using the "right-tailed Fisher z-test". Before treatment, a positive correlation was observed between beta strength and the clinical parameter of FMA in the control group (P = 0.039), while a negative correlation was found between the DAR parameter and the clinical parameter of FMA in the intervention group (P = 0.043). However, no statistically significant correlation between EEG signal rehabilitation parameters and the clinical parameter of FMA was observed in any of the three rehabilitation sessions.
After therapy, a significant negative correlation between pdBSI and FMA was observed in the control group. Additionally, the association between the ART and DAR variables was significant in both the control and intervention groups. A positive correlation between the ART and DAR variables, as well as a negative correlation of the ART parameter with DAR, was observed in both groups.
5. Discussion
This study explored the effects of game-based rehabilitation on neurological recovery using EEG signals. The results demonstrated improvements in Fugl-Meyer scores, attributed to a comprehensive rehabilitation strategy that combined motion learning, retraining, and repetitive activities in various settings. The innovative system integrated observational movement, mental imagery, VR, and repeated practice methods, enhancing motor learning outcomes. These findings align with previous research, supporting the effectiveness of game-based interventions in neurological rehabilitation and highlighting the potential of combining multiple therapeutic approaches for improved patient recovery (26).
Clinical assessments conducted post-rehabilitation revealed improvements in both groups; however, the differences were not statistically significant (P > 0.05). The lack of statistical significance may be due to several factors, including insufficient exercise intensity and repetition. Participants identified this as a limitation of the current protocol. The shoulder wheel system, combined with the virtual environment, might have a more pronounced effect on functional assessment outcomes if rehabilitation activities were conducted during the early stages of stroke recovery. However, due to ethical considerations and the chronic phase of the patients' conditions, early intervention was not feasible in this study. Additionally, the nature of the shoulder wheel device may have influenced the results. The system primarily engages the shoulder and elbow joints, with limited involvement of the wrist. Many clinical evaluation tests predominantly assess wrist and finger function, creating a potential mismatch between the intervention’s target joints and the evaluation focus.
Exercising with a shoulder wheel in a virtual environment, followed by voluntary workouts, can enhance and extend the immediate benefits of therapy. This learning process involves repeated practice, leading to neuronal changes such as improved nerve-to-muscle connections and increased neurotransmitter activity at the nerve-muscle junction (27).
In the first and third sessions, the control group's alpha strength was lower than that of the intervention group. Cortical dysfunction caused by stroke lesions may impede alpha activity, as alpha frequencies originate in the cortical layers. Alpha attenuation has been identified as an indication of brain injury in studies combining EEG, MRI, and CT. In the first and third sessions, beta activity was greater in the control group than in the intervention group. However, previous research has not provided solid evidence linking beta activity to post-stroke pathology. For all sessions, the control group exhibited lower theta activity than the intervention group. In contrast, Kaplan and Rossetti's study found theta to be an unreliable predictor of disease following stroke (24, 28, 29).
DAR demonstrated a statistically significant difference between the control and intervention groups in the first and third sessions of this investigation. Across all sessions, there was a weak negative association in both the delta and theta groups. In the intervention group, delta and alpha data from the first session indicated a slight positive correlation. Various studies have identified DAR as the most consistent neurological characteristic for stroke prediction and post-stroke recovery (30). Similarly, DTR has been identified as a possible marker for post-stroke cognitive outcomes (29, 31, 32), with higher theta power linked to better cognitive results (33, 34).
A higher pdBSI value indicates a loss of neurological and electrical balance between the two hemispheres due to a stroke-induced lesion. Previous research has reported elevated pdBSI levels in stroke patients compared to healthy controls (35). However, in this investigation, a significant difference in pdBSI was identified between the control and intervention groups during the third rehabilitation session. This conclusion aligns with othrer findings, which reported low pdBSI levels associated with post-stroke recovery and absence of lesions (36).
DTR, TBAR, and pdBSI demonstrated substantial changes following therapy in the delta intervention group, with these shifts linked to FMA alterations. Overall, these findings suggest that delta band strength and interactions between theta, beta, and alpha fluctuations could serve as biomarkers due to the pre-existing association between clinical measures (Fugl-Meyer and ART) and EEG signal parameters such as pdBSI and DAR. Although more research is needed, the foundation of EEG signals was suggested for baseline restoration.
The study's findings indicate that this form of rehabilitation system (game-based with EEG signal recording) may be valuable for patients requiring rehabilitation in the future. It is proposed that the protocol explored in this study be applied in future research on the rehabilitation of other body parts, such as the hands, feet, and neck, using similar game-based systems with EEG signal recording. Additionally, it is recommended to integrate games with traditional rehabilitation equipment for various body regions in subsequent investigations.