1. Background
Due to difficulties in early diagnosis, ovarian cancer is associated with a high mortality rate in women (1, 2), with a 5-year survival rate of less than 30% (3, 4). Cisplatin or carboplatin are the most widely used chemotherapeutic agents against ovarian cancer. Ovarian cancer inevitably acquires resistance to these drugs when cancer cells become insensitive to the effects of platinum-based chemotherapy, leading to treatment failure and disease progression. Cancer recurrence after initial chemotherapy is very common in this cancer. One of the challenging issues in the successful treatment of ovarian cancer is the development of drug resistance (5-9).
Sortilin 1 (SORT1) is a multifunctional protein involved in cancer development. The expression of the SORT1 gene is increased in human tumors, including ovarian cancer, and its siRNA-mediated gene silencing in ovarian cancer cells leads to increased apoptosis and decreased proliferation. This suggests that SORT1 is a potential therapeutic target for tumor therapy. Some studies have shown that inhibition of SORT1 expression and function can slow the growth of tumors (10).
Methyl protodioscin, an herbal bioactive compound, was extracted from the rhizome of Dioscorea collettii var. hypoglauca (Dioscoreaceae) and shows some medicinal potential. Its anticancer activities were demonstrated in in vitro and in vivo studies. However, the underlying anti-cancer mechanisms remain unknown. Additionally, a preclinical pharmacodynamic study showed that 80 mg/kg i.v. of methyl protodioscin had no serious side effects (11).
2. Objectives
Considering the necessity of identifying new treatment methods, this study was designed to determine the effects of methyl protodioscin on SORT1 gene expression and the response to chemotherapy in ovarian cancer cells.
3. Methods
3.1. Cell Culture
The A2780s cell line was prepared from the Pasteur Institute, Iran, and cultured in RPMI-1640 medium (Gibco, Germany) containing 10% fetal bovine serum (FBS) and 1% antibiotic. The cells were maintained in a 37°C incubator with 5% CO2. Passage was performed after the cells reached a concentration level of 70%.
3.2. Viability Measurement
The cells were cultured in 96-well plates and treated with concentrations of 0, 0.09, 0.18, 0.37, 0.75, 1.5, 3, 6, and 12 µM methyl protodioscin for 24, 48, 72, and 96 hours. For the trypan blue assay, after trypsinizing and separating the cells from the surface, the cell suspension (about 106 cells/mL) was mixed (ratio of 1:1) with trypan blue (Sigma, USA) 4% solution. After 2 minutes, cells were checked using a neobar lam and an inverted phase optical microscope. Living cells have clear cytoplasm, while dead cells have dark blue cytoplasm. The percentage of cell viability was calculated by dividing the number of unstained cells by the total number of cells.
For the MTT assay, about 10 µL of MTT solution (0.5 mg/mL) (Sigma, USA) was added to each well and incubated for 3 hours in the dark at 37°C. Then, DMSO (200 µL) was added to each well, and the absorbance of the wells was read at 570 nm by an ELISA reader. A row of plate wells without drugs was considered as a control. The viability percentage was calculated from the following equation:
To investigate the effect of methyl protodioscin on carboplatin toxicity, cells were first treated with 800, 400, 200, 100, 50, 25, 12.5, and 6.5 µM of carboplatin for 24 hours. After measuring viability and calculating the IC50 using GraphPad Prism 6.0 (GraphPad Software, USA), combined treatment was performed at IC50 values and two concentrations higher and two lower than the IC50. CompuSyn software (ComboSyn Inc, USA) was used to analyze the cytotoxicity data to determine synergy, additivity, and antagonism between methyl protodioscin and carboplatin.
3.3. Cytotoxicity Assay
The cells were cultured in 96-well plates and treated according to the procedures described in the viability assay. Cytotoxicity testing was performed using the Pierce LDH assay kit (Sigma Aldrich, USA) according to the manufacturer’s instructions. The cytotoxicity of the extract was calculated using the following formula.
3.4. Gene Expression Analysis
After treating the cells and collecting them with trypsin/EDTA solution (Gibco, Germany), RNA extraction was performed according to the TRIZOL reagent kit (Life Technologies Invitrogen, USA). For the quantitative analysis of the extracted RNA, the optical absorption ratio of 260/280 and 230/260 nm was obtained using a NanoDrop. The quality of RNA was tested with agarose gel electrophoresis. The cDNA synthesis was done according to the kit protocol (Bioneer, Korea), and the quality of the synthesized cDNA was verified using a nanodrop device. For real-time PCR, SYBR Green master mix (Takara, Japan) was used. Gene expression was measured by the 2-ΔΔCt method. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. The sequence of primers used is as follows.
(1) Glyceraldehyde 3-phosphate dehydrogenase:
- Forward: TCCTCCACCTTTGACGCTG
- Reverse: CACCACCCTGTTGCTGTAGC
(2) Sortilin 1:
- Forward: TCAGAGCCGAATGCCGTAG
- Reverse: CCTTCCAGCATCTTTGTCCAG
3.5. Statistical Analysis
Data were shown as mean ± SE of three independent tests (each conducted three times, with each time in technical triplicate). Statistical analysis was conducted using SPSS software version 16.0 (SPSS Inc., USA) and a one-way ANOVA test. P-values below 0.05 were considered statistically significant.
4. Results
4.1. Methyl Protodioscin Effects on Cell Viability
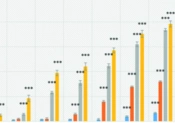
After treatment, the morphology of cells changed, becoming small, round, and granular. The effects of different concentrations of methyl protodioscin on cell viability after 24, 48, 72, and 96 hours were tested using trypan blue and MTT assays, as shown in Figure 1A and B. Treatment with methyl protodioscin significantly decreased viability after 24 hours at concentrations of 0.75, 1.5, 3, 6, and 12 μM (P < 0.05). After 48, 72, and 96 hours, the reduction in cell viability was significant at all concentrations (P < 0.05). This decrease occurred in a concentration- and time-dependent manner. The IC50 values calculated in the trypan blue test were 7.17 ± 0.12, 3.16 ± 0.17, 0.99 ± 0.08, and 0.31 ± 0.05 μM, and in the MTT test were 14.58 ± 0.55, 4.05 ± 0.01, 0.75 ± 0.08, and 0.38 ± 0.01 μM for 24, 48, 72, and 96 hours, respectively.
4.2. Cytotoxic Effects of Methyl Protodioscin
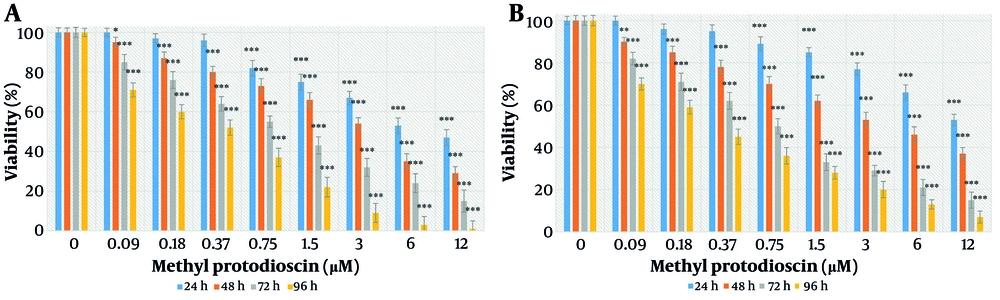
Methyl protodioscin exhibited cytotoxicity against ovarian cancer cells in a concentration- and time-dependent manner. After 24 hours, significant toxicity was observed at concentrations of 3, 6, and 12 μM. After 48 hours, significant toxicity was observed at concentrations of 0.75, 1.5, 3, 6, and 12 μM. After 72 hours, significant toxicity was observed at concentrations of 0.18, 0.37, 0.75, 1.5, 3, 6, and 12 μM. After 96 hours, significant toxicity was observed at all concentrations used (P < 0.05) (Figure 2).
4.3. Methyl Protodioscin Effects on Sortilin 1 Gene Expression
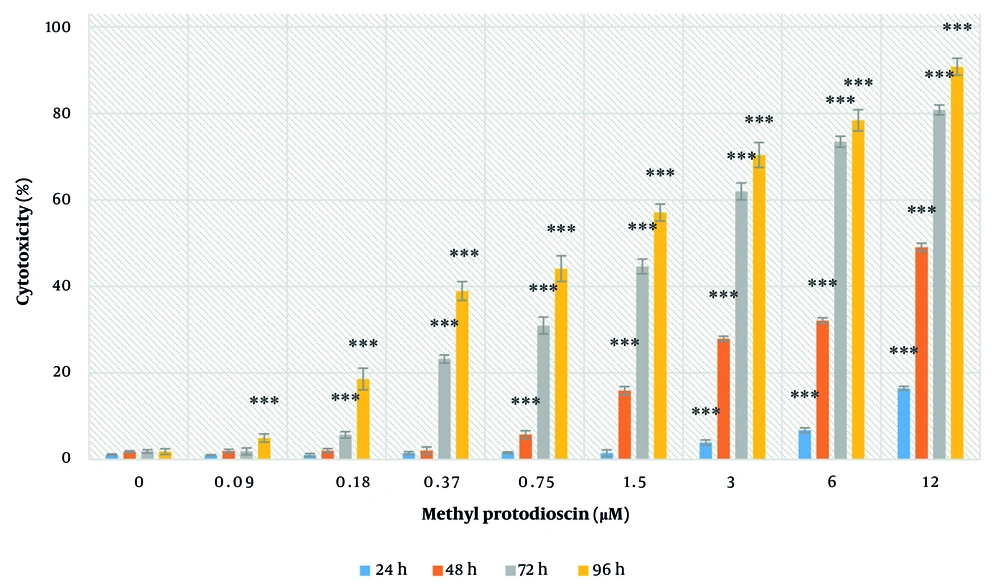
Treatment with the IC50 concentration of methyl protodioscin (14.5 µM) after 24 hours caused a significant decrease in SORT1 expression in ovarian cancer cells by 33% (P < 0.05) (Figure 3).
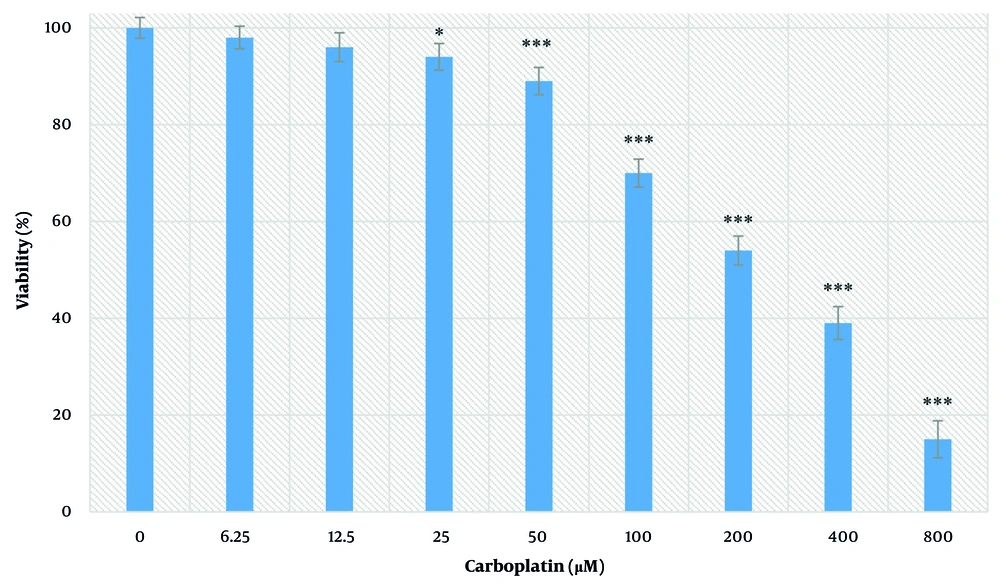
4.4. Carboplatin Effect on Cell Viability
The effects of different concentrations of carboplatin on cell viability after 24 hours were measured by the MTT test and are shown in Figure 4. At concentrations of 25, 50, 100, 200, 400, and 800 µM, a significant decrease in viability was observed (P < 0.05). This decrease occurred in a concentration-dependent manner. The IC50 value was determined to be 228.90 ± 16.10 μM.
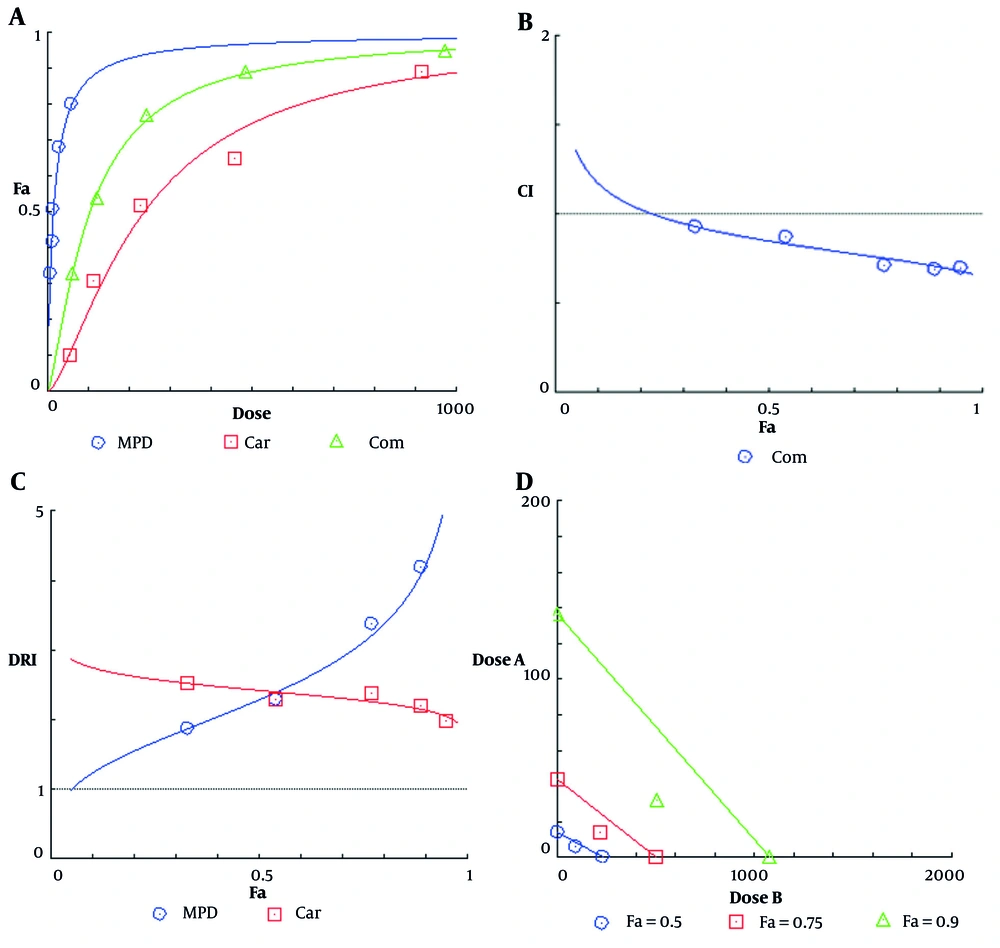
4.5. Carboplatin and Methyl Protodioscin Combination Effect on Cell Viability
The dose-effect diagram showed that the combination of these two agents has more toxic effects on cancer cells than either one alone. The Combination Index (CI) values calculated for all five treatments were less than 1, indicating a synergistic interaction between carboplatin and methyl protodioscin in reducing cell viability. Dose Reduction Index (DRI) values for both carboplatin and methyl protodioscin were greater than 1, indicating a dose reduction to produce a specific therapeutic effect in both cases. Finally, the isobologram plot was drawn. In the isobologram diagram, the concentrations of carboplatin and methyl protodioscin alone and the simultaneous treatment that cause a decrease of 50, 75, and 90 percent of the cell population are shown. In this diagram, the placement of compound points on the chord, below, and above it respectively indicates additive, synergistic, and antagonistic interactions (Figure 5).
Diagram of A, effect-dose; B, Combination Index (CI); C, Dose Reduction Index (DRI); and D, isobologram for ovarian cancer cells after 24 hours of treatment with carboplatin, methyl protodioscin and a combination of two agents. Dose A: Carboplatin concentration, and dose B: Methyl protodioscin concentration.
5. Discussion
The results of the present study showed that methyl protodioscin had a lethal effect on ovarian cancer cell lines. By increasing the concentration of methyl protodioscin, the percentage of cell viability decreased significantly. Additionally, with the increase in the duration of treatment, the percentage of live cells decreased significantly compared to the control group. Previous studies have shown that dioscin had significant inhibitory effects on HL-60 human leukemia cell growth, differentiation, and apoptosis. Moreover, dioscin was found to affect many cancer cells (12). Methyl protodioscin also showed cytotoxic effects on solid tumor cell lines. An analysis utilizing the computer program COMPARE indicated that no compounds in the NCI Anticancer Drug Screen database have similar cytotoxicity patterns to methyl protodioscin, suggesting a potential new mechanism of anticancer action (13). Methyl protodioscin exhibited anti-mitotic activity and induced G2/M arrest and apoptosis in HepG2 cells via cyclin B1 and Bcl-2 expression reduction and Bax expression induction (14). Treatment of oral cancer cells with methyl protodioscin induced apoptosis and could also induce autophagy (15). Protodioscin inhibits cell viability and induces mitochondrial loss of function in apoptosis (16). Methyl protodioscin inhibits proliferation and increases apoptosis in pancreatic cancer (11). In cervical cancer cells, methyl protodioscin induces cell cycle arrest and apoptosis (17).
Despite recent advancements in cancer therapy, the life span and quality of life of patients with ovarian cancer remain unsatisfactory due to the development of drug resistance. Thus, developing new strategies to overcome this problem is needed. Recently, the use of drug combinations has been employed for lethal diseases such as cancer. The major purpose of this strategy is to achieve a synergistic therapeutic effect, reduce the dosage and toxicity, and minimize or delay the development of drug resistance. Today, the use of several anticancer drugs from different groups is widely practiced in cancer treatment (18).
Methyl protodioscin significantly increases the cytotoxicity of carboplatin, with a CI between 0.69 and 0.92, indicating a synergistic effect across all combined concentrations used in this project. The average CI for all tests performed was 0.87, highlighting the overall synergistic effect of the combination of methyl protodioscin and carboplatin on the ovarian cancer cell line. This combination led to a dose reduction for carboplatin, which is clinically valuable as it reduces the general side effects of chemotherapy. Real-time PCR results showed that treatment of cancer cells with the IC50 concentration of methyl protodioscin for 24 hours significantly reduced SORT1 gene expression compared to the control group.
The first molecular characterization of SORT1 showed that it is expressed in the heart, brain, placenta, skeletal muscle, testis, thyroid, and spinal cord (19). The SORT1 gene expression was increased in ovarian cancer tissues compared to non-malignant ovarian tissues (20, 21). These results may suggest the possible role of SORT1 in ovarian tumorigenesis. The potential role of overexpressed SORT1 in ovarian cancer cells is an interesting topic for research. It has previously been proven that decreased SORT1 expression in ovarian tumors leads to apoptosis induction and reduced proliferation of cancer cells, so its decreased expression induced by methyl protodioscin in this study may be responsible for its anti-ovarian cancer effect.
One of the limitations of this study is the lack of examination of SORT1 siRNA/overexpression experiments to validate its functional role in methyl protodioscin-induced chemosensitization. Currently, it is unknown whether methyl protodioscin synergizes with carboplatin in SORT1-silenced cells.
5.1. Conclusions
Methyl protodioscin had an inhibitory effect on the viability of ovarian cancer cells in a concentration- and time-dependent manner and reduced the expression of the SORT1 gene. The present study also showed that this herbal compound synergistically enhances the chemotherapy effects of carboplatin. The results suggest that methyl protodioscin has a therapeutic effect on ovarian cancer cells and holds promise for use in the development of anti-ovarian cancer drugs.