1. Background
The healthy state of the body depends on the precise functioning of the liver for waste removal and xenobiotic metabolism, and its dysfunction by toxic chemicals leads to serious health problems. Liver dysfunction due to inhalation or ingestion of hepatotoxic substances such as acetaminophen, cadmium chloride, ethanol, carbon tetrachloride, and alcohol is increasing significantly worldwide (1). Some medicinal plants have shown hepato-protective activity (2, 3). For evaluating plants with hepato-protective effects, laboratory assays can be used. Hepatoma cell lines can serve as substitutes for hepatocytes in in vitro models of liver cells (4). The HepG2 cell line is widely used in the investigation of liver function, metabolism, and drug toxicity (5, 6). It also has many biochemical and morphological characteristics of normal hepatocytes (7). Therefore, it can be used in investigations about the hepato-protective activity of medicinal plants (8, 9). The most important inducers of hepato-toxicity are paracetamol (acetaminophen) and carbon tetrachloride (10). The mechanisms responsible for their hepatotoxicity are complex. Carbon tetrachloride undergoes metabolic activation and generates free radicals, which are responsible for its toxicity (11).
Taraxacum officinale (Dandelion) is a plant of the Taraxacum genus and the Asteraceae family that has been used in traditional medicine since ancient times. This plant is cultivated in fields for medicinal and food purposes mainly in some countries. In the traditional medicine of Russia, India, and China, dandelion is mentioned because of its effects on human health. In these regions, this substance is often consumed as a food (salad) and is a rich source of micronutrients such as minerals and vitamins. In traditional medicine, many therapeutic benefits of this plant have been mentioned, including the treatment of diabetes, blisters, liver problems, cardiovascular diseases, and hyperlipidemia. In previous studies, the hepato-protective effect of dandelion has been investigated in vivo (2).
2. Objectives
Considering the importance of understanding the mechanism of the effect of substances in the development of new treatment methods, in this study, the mechanism of the protective effect was investigated in vitro.
3. Methods
3.1. Cell Line and Reagents
HepG2 cell line was purchased from the national cell bank of Iran (NCBI). All materials used in this study was purchased from Gibco Company (USA).
3.2. Extraction
The plant materials were collected from around Kermanshah (located in the western part of Iran) in early spring 2020, and their impurities were removed. After confirmation by the botanist, the hydroalcoholic extract was prepared as described previously (12).
3.3. Cell Culture
The cells were seeded in DMEM containing 10% FBS without antibiotics at 37°C with 5% CO2 in a humidified atmosphere.
3.4. Dose Optimization of Extract
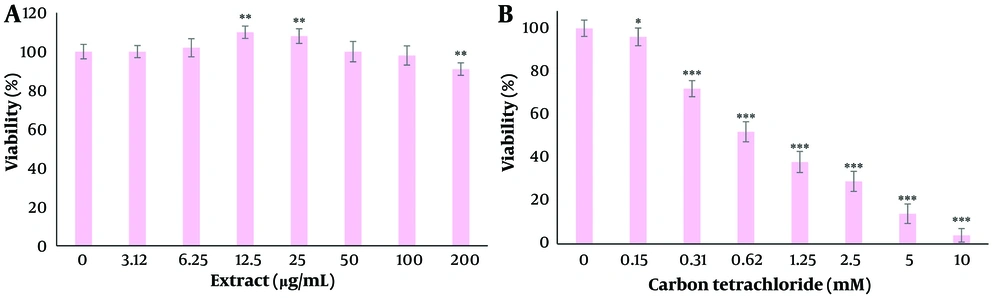
To select non-toxic concentrations, cells were treated with 200, 100, 50, 25, 12.5, 6.25, and 3.12 µg/mL of extract for 24 hours, and viability was estimated by the MTT technique as described previously (12).
3.5. Dose Optimization of Vancomycin
To determine the IC50 value, the cells were treated with 10, 5, 2.5, 1.25, 0.62, 0.31, and 0.15 mM of carbon tetrachloride and incubated for 24 hours, and viability was estimated by the MTT technique as described previously (12). The IC50 value was obtained using GraphPad Prism 5 software (GraphPad Software Inc, USA).
3.6. Effect of Dandelion Extract on Carbon Tetrachloride-Induced Hepatotoxicity
To study the effect of the extract on the cytotoxicity caused by carbon tetrachloride, the cells were treated with non-toxic concentrations of the extract and the IC50 value of carbon tetrachloride. Three types of experiments were performed as follows:
(a) In the pre-treatment study, the cells were first treated with the extract for 24 hours and then with carbon tetrachloride, followed by incubation for another 24 hours. Finally, cell viability was determined by the MTT assay.
(b) In the co-treatment study, the cell line was treated with the extract and carbon tetrachloride simultaneously for 24 hours. Then, the viability was estimated by the MTT method.
(c) In the post-treatment study, the cell line was treated with carbon tetrachloride for 24 hours and then with the extract, followed by incubation for another 24 hours. The MTT assay was performed.
3.7. Effect of Dandelion Extract on Carbon Tetrachloride-Induced Apoptosis
The percentage of DNA fragmentation after 24 hours of treatment with the extract and/or carbon tetrachloride was determined using the diphenylamine method described in previous studies by Cohen and Duke (as cited by Pahlavani and Vargas) (13), and the absorbance of the samples was determined using a spectrophotometer at 600 nm. Apoptosis was also quantified using the Annexin V-FITC Apoptosis Staining/Detection Kit (ab14085) according to the manufacturer's instructions. Additionally, apoptotic markers were investigated by real-time PCR test. Briefly, RNA was extracted using the TRIzol reagent (Thermo Fisher Scientific, USA), and its purity was determined at 260/280 nm. Single-stranded complementary DNA (cDNA) was produced using a cDNA synthesis kit supplied by Vivantis Technologies (Selangor DE, Malaysia) according to the manufacturer’s protocol. Amplification of cDNA was performed using SYBR Green master mix supplied by Thermo Scientific (MA, USA). GAPDH was used as an internal standard gene, against which cDNA was normalized. Real-time PCR conditions were: 95°C for 10 minutes and 40 cycles of 95°C for 15 seconds followed by 60°C for 1 minute. The primer sequences were as follows:
- Bax: Forward: 5´-CCTGTGCACCAAGGTGCCGGAACT-3´; Reverse: 5´-CCACCCTGGTCTTGGATCCAGCCC-3´
- Bcl-2: Forward: 5´-TTGTGGCCTTCTTTGAGTTCGGTG-3´; Reverse: 5´-GGTGCCGGTTCAGGTACTCAGTCA-3´
- GAPDH: Forward: 5´-TCCCTGAGCTGAACGGGAAG-3´; Reverse: 5´-GGAGGAGTGGGTGTCGCTGT-3´
3.8. Data Analysis
The data were expressed as means ± standard deviation (SD) from three independently repeated tests. Statistical analysis was performed using ordinary one-way ANOVA followed by Tukey post-hoc tests using SPSS software, and P < 0.05 was considered a significant difference.
4. Results
4.1. The Extract and/or Carbon Tetrachloride Effect on Cell Viability
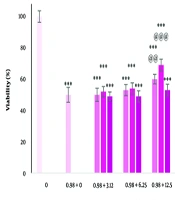
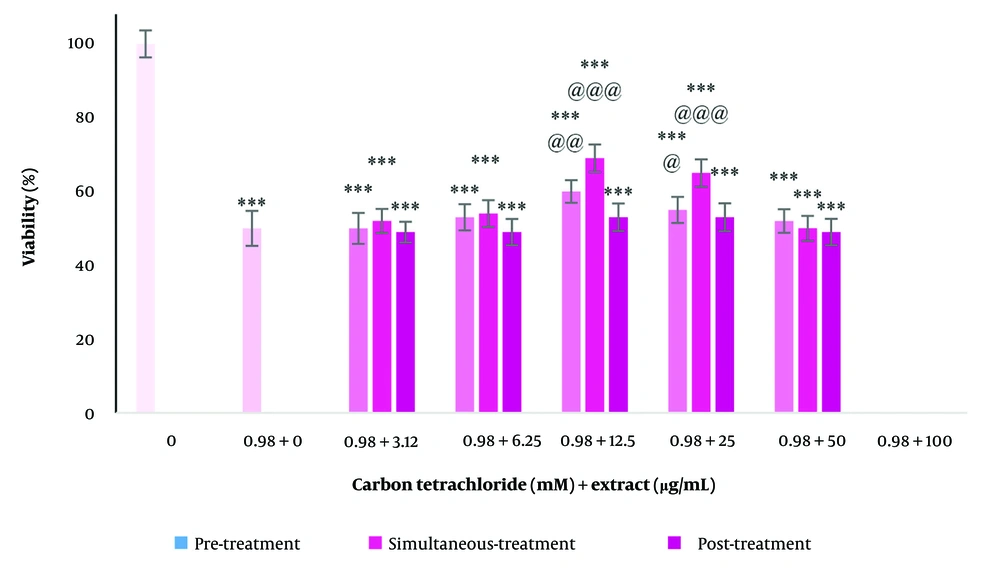
The effects of different concentrations of dandelion extract and carbon tetrachloride on cell viability after 24 hours are shown in Figure 1A and B. There was a significant decrease in cell viability at the concentration of 200 µg/mL (P < 0.05). Additionally, a significant increase was observed at concentrations of 12.5 and 25 µg/mL (P < 0.05). After treatment with carbon tetrachloride, cell viability was significantly decreased in a concentration-dependent manner (P < 0.05). The IC50 value for carbon tetrachloride was 0.98 µg/mL for 24 hours. Only pre-treatment and co-treatment at concentrations of 12.5 and 25 µg/mL of the extract had a significant effect on reducing the toxicity of carbon tetrachloride (P < 0.05). The greatest effect was observed in co-treatment at a concentration of 12.5 μg/mL extract (Figure 2).
The effect of pre-treatment, simultaneous treatment and post-treatment with different concentrations of dandelion extract and carbon tetrachloride on the viability of liver cells. *** P < 0.001 compared to the control cells and @ P < 0.05, @@ P < 0.01 and @@@ P < 0.001 compared to the carbon tetrachloride treated cells.
4.2. The Effect of Dandelion Extract on Carbon Tetrachloride-Induced Apoptosis
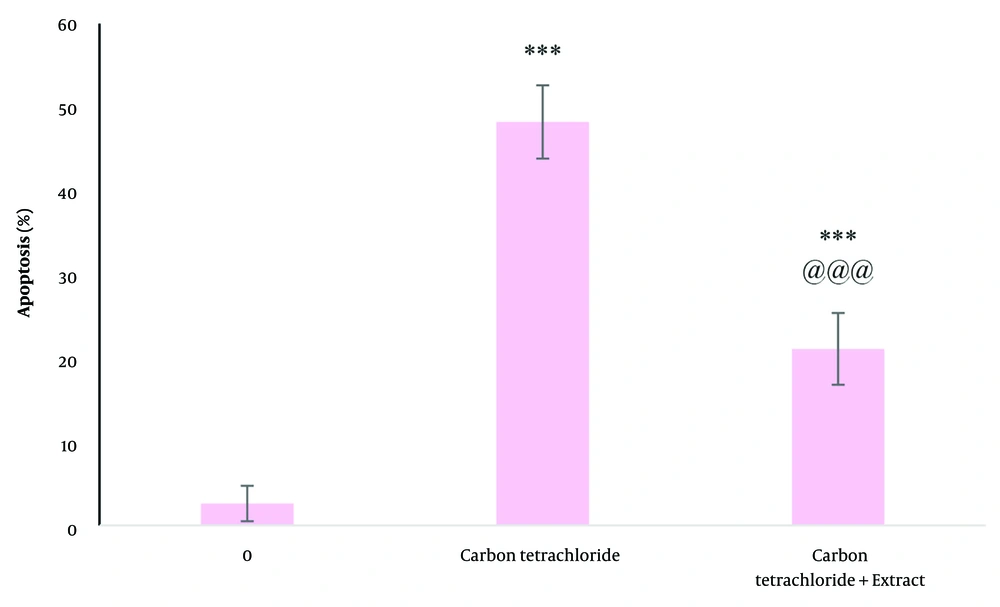
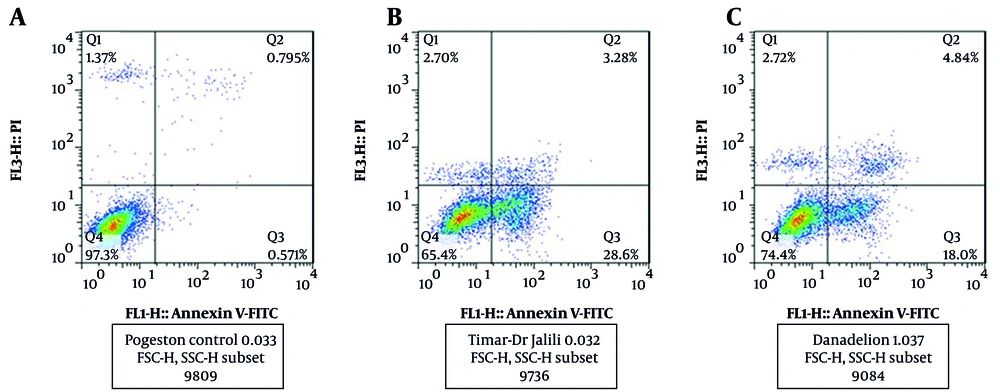
Cell apoptosis significantly increased with treatment with carbon tetrachloride (P < 0.05). Dandelion extract significantly reduced apoptosis induced by carbon tetrachloride (P < 0.05) (Figure 3). Apoptosis measurement by flow cytometry showed that in the control group, 97.3% of cells were alive, 0.57% were in the early stage of apoptosis, 0.79% were in the late stage of apoptosis, and 1.37% were necrotic. In the cells treated with carbon tetrachloride, 65.40% of the cells were alive, 28.60% were in the early stage of apoptosis, 3.28% were in the late stage of apoptosis, and 70.2% of the cells were necrotic. In cells treated with dandelion extract and carbon tetrachloride, 74.40% of cells were alive, 18% had early apoptosis, 4.84% had late apoptosis, and 72.2% had necrosis (Figure 4).
The effect of carbon tetrachloride and carbon tetrachloride + dandelion extract on the apoptosis in liver cells after 24 (h) of treatment was investigated by diphenylamine test. *** P < 0.001 compared to the control cells and @@@ P < 0.001 compared to the carbon tetrachloride-treated cells.
4.3. The Effect of Carbon Tetrachloride and Dandelion Extract on the Expression of Bax and Bcl-2 Genes
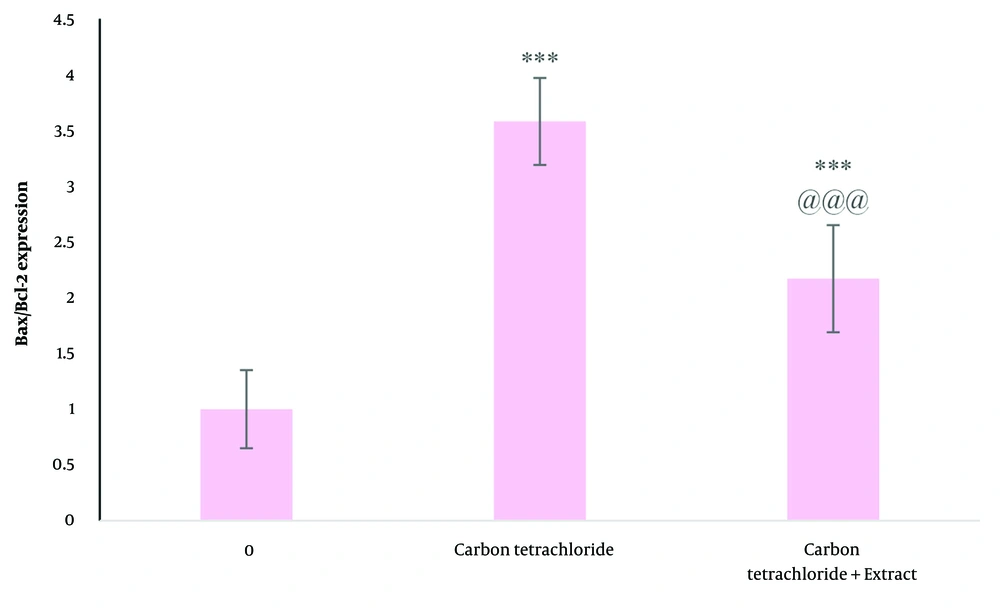
Analysis of gene expression showed that carbon tetrachloride treatment significantly decreased Bcl-2 gene expression (P < 0.05). Bax gene expression showed a significant increase after treatment (P < 0.05). Thus, dandelion plant extract decreased the Bax/Bcl-2 ratio compared to carbon tetrachloride-treated cells (Figure 5).
The effect of carbon tetrachloride and carbon tetrachloride + dandelion extract on the Bax/Bcl-2 expression in liver cells after 24 (h) of treatment was investigated by real-time PCR test. *** P < 0.001 compared to the control cells and @@@ P < 0.001 compared to the carbon tetrachloride-treated cells.
5. Discussion
In this study, the effect of dandelion extract on the viability of liver cells was first tested. The data indicated that after a 24-hour period of treatment, the viability of cells at a concentration of 200 µg/ml decreased significantly. Additionally, after 24 hours of treatment with carbon tetrachloride, the decrease in viability was concentration-dependent. The IC50 was calculated for carbon tetrachloride. In the subsequent tests, the IC50 concentration was used along with non-toxic concentrations of the extract. The results of the co-treatments showed that the simultaneous use of the extract at a concentration of 12.5 μg/mL and carbon tetrachloride had the greatest protective effect. Some studies have investigated the protective effect of different dandelion plant extracts or components isolated from it in in vivo conditions. In these studies, different toxic agents were used, including carbon tetrachloride, acetaminophen, alcohol, sodium dichromate, and digalactosamine (14-33). All these studies confirmed the protective effect of dandelion. Data from this study showed that carbon tetrachloride exerted its toxic effects by inducing apoptosis in liver cells. Dandelion showed a protective effect by reducing apoptosis induced by carbon tetrachloride. This effect of dandelion plant extract is probably due to its antioxidant property. Numerous studies have confirmed the antioxidant properties of dandelion in different geographical areas. Reactive oxygen species (ROS) and the resulting oxidative stress play an essential role in apoptosis. Antioxidants can inhibit the apoptosis process. Bcl-2 prevents apoptosis. The ROS and the resulting oxidative stress induce apoptosis. Increasing evidence shows that oxidative stress and apoptosis are involved in the pathophysiology of some chronic diseases (34). There are many different intracellular proteins with anti-apoptotic and pro-apoptotic activity, and the ratio between them plays a critical role in regulating apoptosis. Bcl-2 inhibits apoptosis without affecting cell proliferation, while Bax promotes apoptosis (35). The data from this study showed that carbon tetrachloride increased the ratio of Bax/Bcl-2 in liver cells. Dandelion plant extract reduced this increase to some extent.
5.1. Conclusions
Dandelion extract had a protective effect on liver cells. It can reduce apoptosis induced by carbon tetrachloride via regulation of the expression of the Bax to Bcl-2 ratio.