1. Background
Vancomycin is a commonly used antibiotic against gram-positive bacteria (1, 2). However, bacteria can develop resistance to vancomycin. To overcome this resistance, a high dose of vancomycin is often suggested (3-5). Nevertheless, the use of high doses of vancomycin is associated with (4) a risk of nephrotoxicity (6). Nephrotoxicity occurs in 5 - 25% of vancomycin users and increases to 25 - 35% when vancomycin is used in combination with aminoglycosides (7-9). It has been proposed that oxidative stress, apoptosis, and inflammation are involved in vancomycin-induced nephrotoxicity (8, 10, 11). Oxidative stress is one of the main mechanisms of vancomycin-induced nephrotoxicity (12). It regulates the caspase cascade, resulting in apoptosis (13).
Herbs and their derivatives are popular for their antioxidant properties and may be used to reduce vancomycin-induced nephrotoxicity without diminishing the antibiotic effect. Taraxacum officinale (dandelion), from the Taraxacum genus and Asteraceae family, has been used in traditional medicine since ancient times. This plant is cultivated on farms for medicinal and food purposes, mainly in Bulgaria, Romania, Hungary, and Poland. In traditional Russian, Indian, and Chinese medicine, dandelion is noted for its lipid-lowering effects. In these regions, it is often consumed as a food (salad) and is a rich source of micronutrients such as minerals and vitamins. The therapeutic effect of this plant on various human disorders has been recorded in traditional medicine (14).
2. Objectives
Previous studies have investigated the diuretic and protective effects of dandelion on kidneys in vivo (14). Here, the mechanism of the proposed protective effect of dandelion on kidney cells was investigated in vitro.
3. Methods
3.1. Cell Line and Reagents
The Vero cell line was prepared from the National Cell Bank of Iran (NCBI). Chemical reagents were obtained from Sigma-Aldrich Chemical Co. (USA). The cell culture reagents were purchased from Gibco (Gibco Company, USA).
3.2. Cell Culture
This in vitro study was initiated after approval by the Ethical Committee of Kermanshah University of Medical Sciences, Kermanshah, I.R. Iran (Code: IR.KUMS.REC.1400.090). The cells were seeded in RPMI medium containing 10% fetal bovine serum (FBS) and without antibiotics, maintained at 37°C with 5% CO2 in a humidified atmosphere.
3.3. Preparation of Extract
A hydroalcoholic extract is a solution created by using a mixture of water and alcohol to extract bioactive compounds from plant materials. Dandelion was gathered from around Kermanshah (located in the western part of Iran) in early spring 2020, and its impurities were removed. After approval by a botanist, the hydroalcoholic extract was prepared by maceration using 70% ethanol, as described previously (15).
3.4. Dose Optimization of Extract
The cells were treated with 200, 100, 50, 25, 12.5, 6.25, and 3.12 µg/mL of the extract in 96-well plates (15 × 103 cells/well). After 24 hours, the viability of the cells was estimated using MTT staining, as explained earlier (16).
3.5. Dose Optimization of Vancomycin
Vancomycin was dissolved in a cell culture medium. The cells were treated with 0.15, 0.31, 0.62, 1.25, 2.5, 5, and 10 mg/mL of vancomycin. After 24 hours, the viability of the cells was measured using the MTT test. The IC50 was obtained using GraphPad Prism 8 software (USA).
3.6. Extract Effect on Vancomycin-Induced Nephrotoxicity
For combination treatment, after culturing the cells in 96-well plates, three treatment plans were used. In the pre-treatment plan, the cells were treated with non-toxic concentrations of the extract. After 24 hours, the cells were treated with the IC50 of vancomycin for another 24 hours. For the simultaneous treatment plan, the cells were treated with a combination of the extract and vancomycin for 24 hours. For the post-treatment plan, the cells were treated with the IC50 concentration of vancomycin. After 24 hours, they were treated again with the extract. Finally, the cell viability was tested by MTT staining.
3.7. Effect of Extract on Vancomycin-Induced Apoptosis
The percentage of DNA fragmentation after 24 hours of treatment with vancomycin and extract was tested using the diphenylamine test, as expressed by Cohen and Duke (17). Additionally, for quantitative estimation of cell death, the FITC-Annexin V Apoptosis Detection Kit (BD Bioscience, Heidelberg, Germany) was used according to the manufacturer's instructions. Finally, the cells were analyzed using a flow cytometer (17).
3.8. Molecular Analysis
The apoptosis-related gene expression analysis was performed by real-time PCR. RNA isolation was conducted with 106 cells from both control and treatment groups, using an RNA isolation kit (Roche Diagnostics, Basel, Switzerland). The RNA purity and integrity were checked using a NanoDrop spectrophotometer and gel electrophoresis. Complementary DNA (cDNA) was synthesized according to the cDNA Transcriptor First Strand cDNA synthesis kit procedure (Roche Diagnostics). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Real-time PCR was conducted using SYBR Premix Ex Taq Technology supplied by Takara Bio Inc. (Shiga, Japan). The primers for target and reference genes were purchased from CinnaGen Co. (Tehran, Iran) and were as follows:
- Bax:
- Forward: 5´-CCTGTGCACCAAGGTGCCGGAACT-3´
- Reverse: 5´-CCACCCTGGTCTTGGATCCAGCCC-3´
- Bcl-2:
- Forward: 5´-TTGTGGCCTTCTTTGAGTTCGGTG-3´
- Reverse: 5´-GGTGCCGGTTCAGGTACTCAGTCA-3´
- Glyceraldehyde 3-phosphate dehydrogenase:
- Forward: 5´-TCCCTGAGCTGAACGGGAAG-3´
- Reverse: 5´-GGAGGAGTGGGTGTCGCTGT-3´
3.9. Statistical Analysis
The results were expressed as mean ± standard deviation. The Student's t-test or one-way analysis of variance (ANOVA) with the Tukey Comparison Test as a post-test were used for statistical analysis. A significance value was accepted as P < 0.05.
4. Results
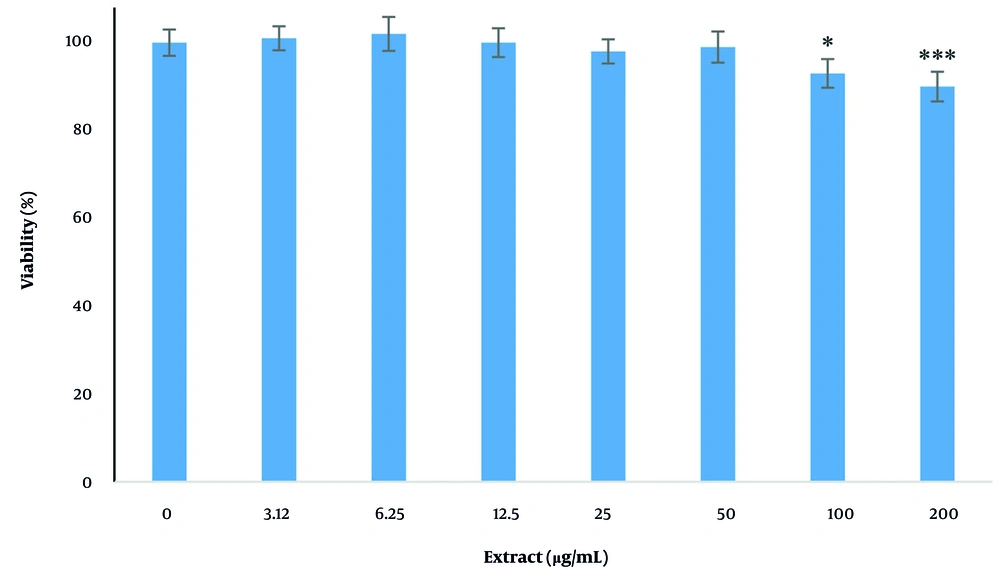
4.1. The Effects of Plant Extract on Cells Viability
The effects of various concentrations of the extract (3.12, 6.25, 12.5, 25, 50, 100, and 200 µg/mL) on the viability of cells after 24 hours are shown in Figure 1. It is evident that the extract significantly reduced the viability of the cells at concentrations of 100 and 200 µg/mL, while lower concentrations were not toxic to the cells (P < 0.05).
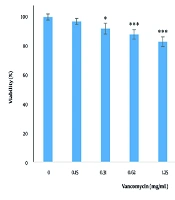
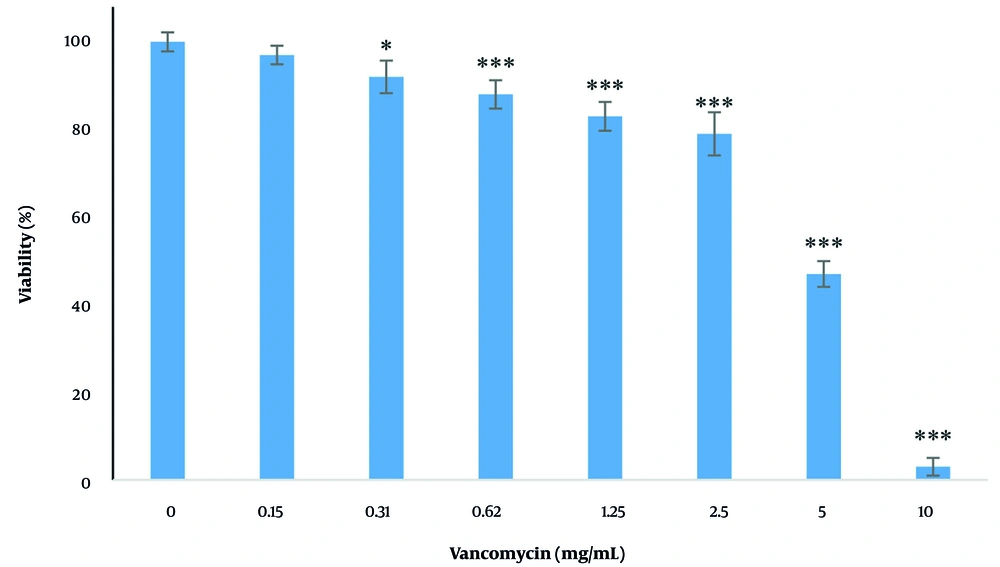
4.2. The Effect of Vancomycin on Cell Viability
The effects of various concentrations of vancomycin (0.15, 0.31, 0.62, 1.25, 2.5, 5, and 10 mg/mL) on the viability of cells after 24 hours are shown in Figure 2. There was a significant decrease (P < 0.05) in cell survival at concentrations of 0.31, 0.62, 1.25, 2.5, 5, and 10 mg/mL, and this reduction occurred in a dose-dependent manner. The IC50 value of vancomycin was determined to be 2.65 mg/mL. For subsequent tests, the IC50 concentration was used.
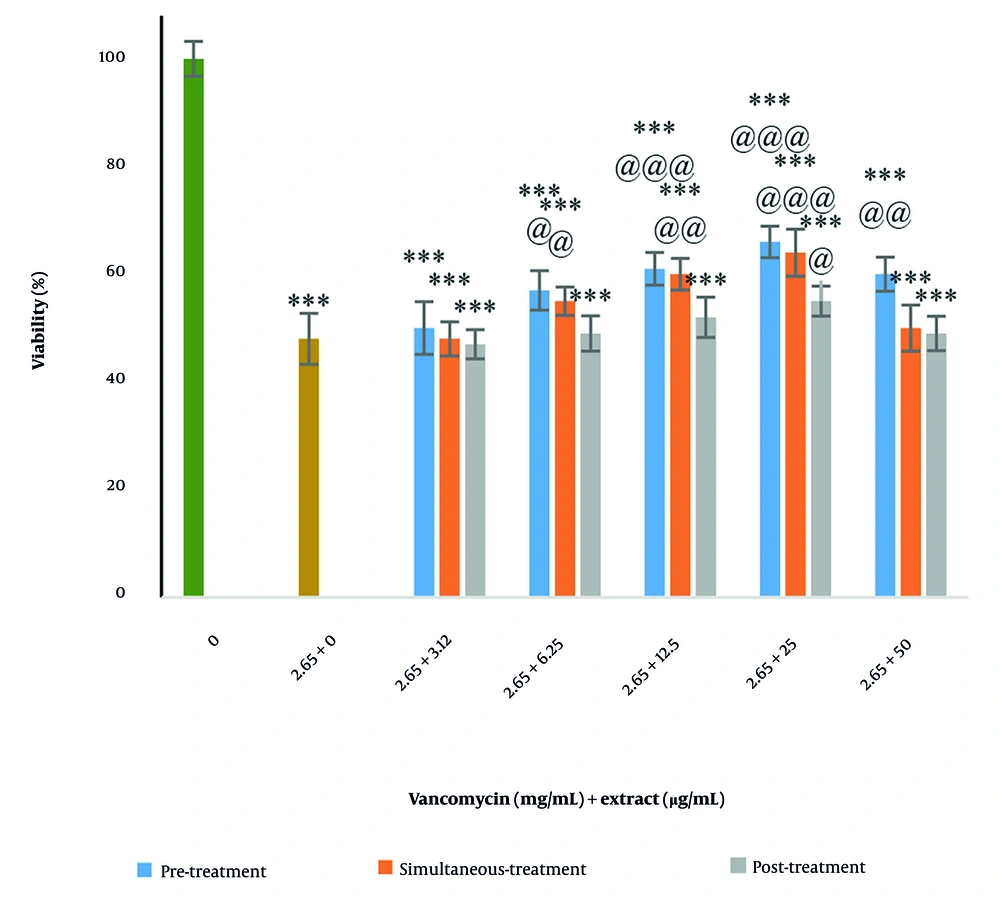
4.3. Effect of Plant Extract on Vancomycin-Induced Cytotoxicity
In the pre-treatment plan, the extract significantly reduced vancomycin cytotoxicity at concentrations of 6.25, 12.5, 25, and 50 µg/mL (P < 0.05). In the simultaneous treatment plan, the extract significantly reduced vancomycin cytotoxicity at concentrations of 6.25, 12.5, and 25 µg/mL (P < 0.05). In the post-treatment plan, the extract significantly reduced vancomycin cytotoxicity at a concentration of 25 µg/mL (P < 0.05). Thus, the extract protected Vero cells against vancomycin cytotoxicity. Among these three treatment plans, pre-treatment was the most effective in protecting cells and was used for other tests (Figure 3).
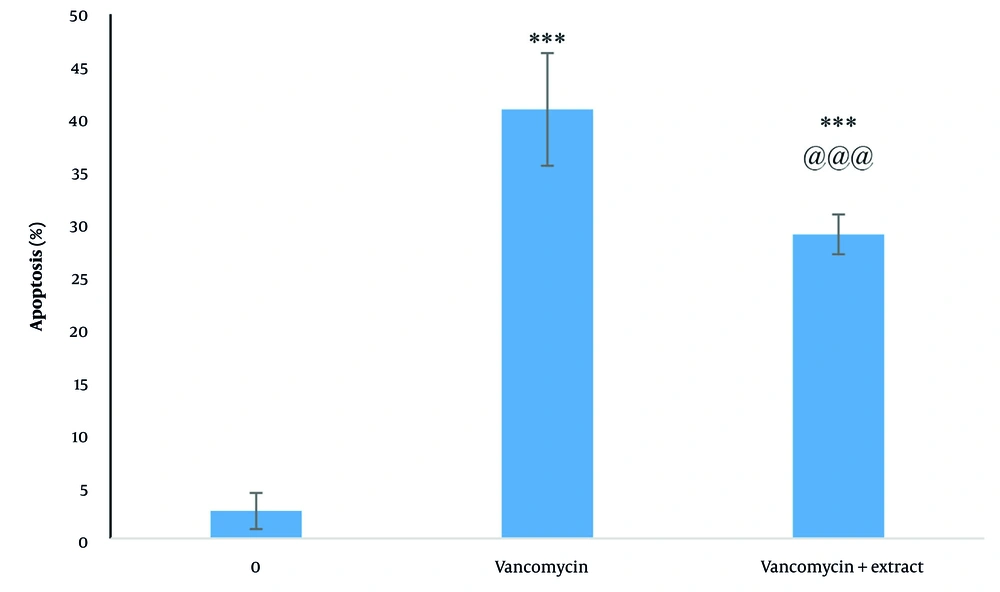
4.4. Effect of Plant Extract on Vancomycin-Induced Apoptosis
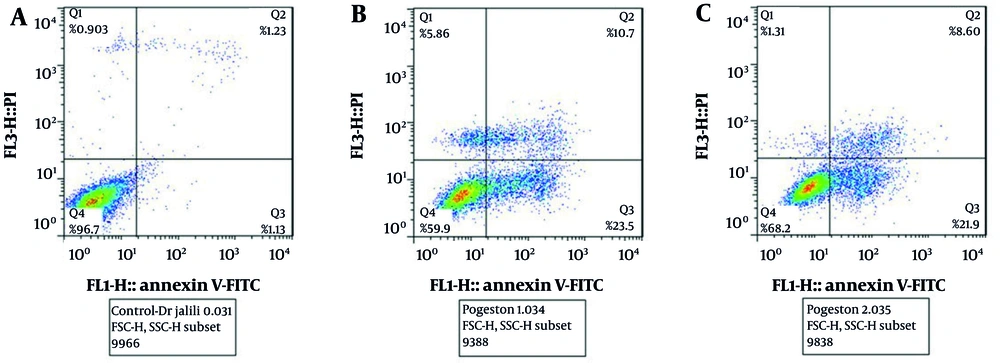
The results indicated that vancomycin significantly increased apoptosis compared to control cells (P < 0.05), with a 15.61-fold increase. The extract significantly reduced apoptosis induced by vancomycin (P < 0.05) (Figure 4). Additionally, the results of Annexin V-FITC staining showed that in the control group, 96.7% of cells were viable, 1.13% were in the early apoptosis stage, 1.12% were in the late apoptosis stage, and 0.90% were necrotic cells. The cells treated with vancomycin showed a significant increase in early apoptotic cells (23.50%), late apoptotic cells (10.70%), and 5.86% were necrotic cells, with a total percentage of apoptotic cells at 34.20%. The extract reduced apoptosis induced by vancomycin, resulting in 68.20% of cells being viable, 21.90% early apoptotic, 8.60% late apoptotic, and 1.31% necrotic cells (Figure 5).
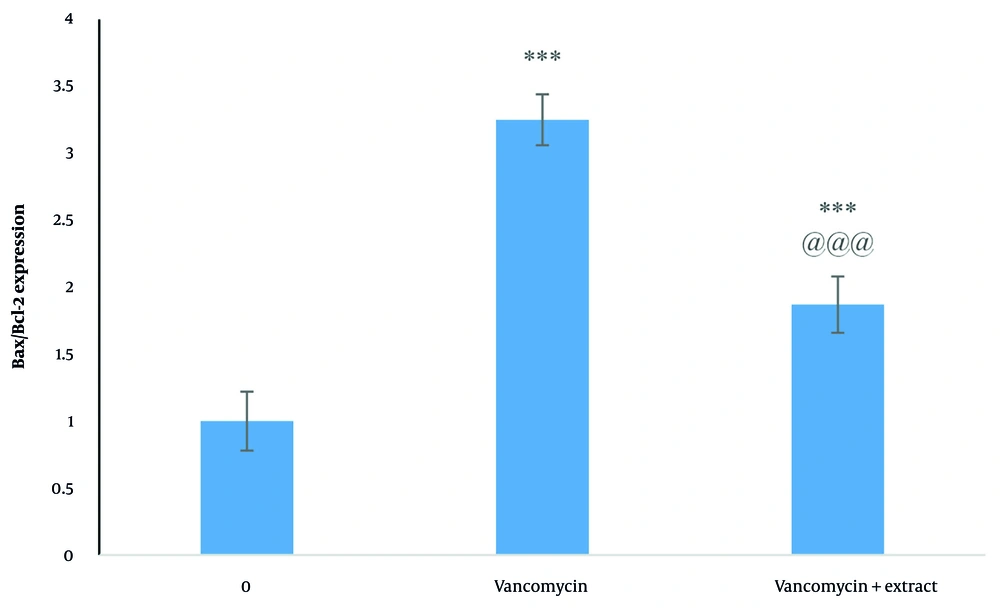
4.5. Gene Expression Analysis
The results of real-time PCR showed upregulated Bax expression and downregulated Bcl-2 expression in vancomycin-treated kidney cells. The extract increased Bcl-2 expression and decreased Bax expression (Figure 6).
5. Discussion
In the present work, the effect of dandelion extract on vancomycin-induced nephrotoxicity was assessed, along with its effect on apoptosis-related cell death. Nephrotoxicity is one of the common complications of vancomycin. Despite recent advances in pharmaceutical research, vancomycin remains the main treatment for methicillin-resistant Staphylococcus aureus and methicillin-resistant S. epidermidis (18). Oxidative stress is one of the main proposed mechanisms for this toxicity (19). Finding a strategy that prevents or reduces the nephrotoxicity of vancomycin can help continue the use of this medicine. The use of herbal medicines has been studied to minimize this complication (20).
The data from this study showed that dandelion extract protected Vero cells against vancomycin cytotoxicity. Among the three treatment plans, pre-treatment was the most effective in protecting cells and was used for other tests. Reactive oxygen species (ROS) accumulation and the consequent oxidative stress in cells can cause apoptosis. Antioxidants can prevent this process. The ROS and the consequent cellular redox modification can be part of the signal transduction pathway involved in apoptosis induction (21). Oxidative stress changes the expression of the apoptosis-related genes Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic). These genes are involved in regulating apoptosis. An increased Bax/Bcl-2 ratio directly corresponds to apoptosis induction (22, 23). Our data revealed that vancomycin-induced nephrotoxicity was accompanied by an increased Bax/Bcl-2 ratio. However, Bax expression was decreased and Bcl-2 expression was increased with dandelion extract treatment. In combination treatment, the extract inhibited vancomycin-induced apoptosis.
Dandelion has a potent antioxidant effect in different tissues, and this plant contains compounds known as highly effective scavengers for ROS. The antioxidant effect of different extracts of dandelion in various geographical regions has been detailed in a review by Jalili et al. (14). The Bax/Bcl-2 shift supports antioxidant-mediated apoptosis inhibition. Thus, dandelion’s known compounds (e.g., luteolin, chicoric acid) may be responsible for apoptosis inhibition.
Vancomycin causes interstitial edema, tubular dilatation, tubular epithelial cell desquamation, and vacuolization in the kidneys of rats (24-26). In the present in vitro study, we showed that vancomycin exposure caused apoptosis in kidney cells, and dandelion attenuated these unwanted toxic effects. Vancomycin treatment decreased the viability of cells in a dose-dependent manner. Our investigations were conducted with 2.65 mg/mL vancomycin, which induced moderate cell death (IC50). Dandelion extract pre-treatment markedly reduced the cytotoxic effects of vancomycin. This effect is likely due to the antioxidant activity of dandelion. Studies have revealed that oxidative stress plays a significant role in the pathogenesis of nephrotoxicity both in vivo and in vitro (27-30).
In a similar study, the protective effects of dandelion on carbon tetrachloride-induced kidney damage were examined in female Wistar albino rats. This study showed that dandelion extract reversed the histopathological changes in the kidney caused by carbon tetrachloride, which was supported by biochemical parameters (31). This protective effect is possibly due to the enhancement of the antioxidant defense system by dandelion extract (32). One limitation of this study is the lack of HPLC characterization of bioactive compounds (e.g., taraxasterol, luteolin), which is suggested for future studies.
5.1. Conclusions
We provide evidence that dandelion extract ameliorates vancomycin-induced toxicity by inhibiting apoptosis. Therefore, the use of dandelion in combination with vancomycin may present a favorable approach to preventing nephrotoxicity. Further in vivo, preclinical, and clinical studies are recommended in this area.