1. Background
Transfusion is a common treatment for critically ill patients to address conditions that cause severe morbidity or death and cannot be avoided or treated successfully by any other means (1). While blood transfusions can save lives, they can also cause dangerous side effects. Any basic healthcare delivery system must provide appropriate and secure blood transfusion services.
A transfusion reaction (TR) is described as any unfavorable occurrence that occurs in a patient during or after the transfusion of blood and blood components for which no other cause can be identified. These negative consequences range in severity from moderate to severe. Acute TRs (ATRs) emerge within 24 hours of transfusion, with the majority occurring during or within four hours (2). Complications associated with blood transfusion treatment can be classified into acute and late TRs, as well as immunological and non-immunological etiologies (3).
Acute immunologic reactions, such as allergic, anaphylactic, transfusion-related acute lung injury (TRALI), acute hemolytic TR (AHTR), and febrile non-hemolytic TR (FNHTR), are all correlated with an immune response to antigens on white cells, red cells, platelets, or plasma proteins (2). According to recent estimates, ATRs occur in 0.2 - 10% of blood transfusions and cause mortality in around 1 out of every 250,000 units. The type of ATR manifested depends on the blood product transfused, the health status of the recipient, and the recipient's previous medical history.
A successful approach to minimizing transfusion-related adverse effects involves judicious patient selection paired with pragmatic pretransfusion evaluations of risk and benefit to the prospective recipient, as well as rigorous quality control. Furthermore, ongoing monitoring of transfusion-related complications will help patients receive better treatment and enhance their safety. The goal of hemovigilance is to monitor, detect, and prevent the occurrence or recurrence of transfusion-related adverse reactions to improve the safety, effectiveness, and performance of the blood transfusion process from donors to recipients (4).
High-quality data are scarce on the advantages and drawbacks of various blood product transfusion procedures used worldwide (5). Knowledge of the different forms of blood TRs can aid not only in early detection and control but also in taking appropriate preventative steps. Since there is no proper and strict hemovigilance system in place throughout the country, it is difficult to ascertain the true frequency of these reactions.
Hemovigilance is a cornerstone of transfusion safety and refers to a set of surveillance procedures covering the entire transfusion chain, from blood donation to the follow-up of recipients, with the aim of collecting and analyzing data on adverse transfusion events (Bolton-Maggs and Cohen). Through comprehensive monitoring and systematic reporting of adverse reactions, hemovigilance systems play a critical role in improving patient safety and refining clinical protocols. Studies from national hemovigilance programs, such as the UK’s Serious Hazards of Transfusion (SHOT), have demonstrated how structured surveillance can lead to significant reductions in preventable transfusion-related complications and improve clinical awareness. Therefore, establishing or enhancing hemovigilance mechanisms in all healthcare settings is essential to ensuring safe transfusion practices and minimizing patient risk (2).
High-quality data are scarce on the advantages and drawbacks of various blood product transfusion procedures used worldwide (3). Knowledge of the different forms of blood TRs can aid not only in early detection and control but also in taking appropriate preventative steps. Since there is no proper and strict hemovigilance system in place throughout the country, it is difficult to ascertain the true frequency of these reactions.
Given the geographical, demographic, and healthcare infrastructure differences across regions, the incidence and types of adverse TRs may vary. Therefore, conducting regional studies is crucial to accurately identify local patterns and risk factors, which can improve transfusion safety and help develop effective hemovigilance systems tailored to each population (4, 5).
2. Objectives
The primary objective of this retrospective study was to investigate the incidence and types of adverse blood TRs at Imam Reza Hospital, a tertiary care center in Kermanshah, Iran. The study aimed to classify the reported reactions, identify their frequency, and evaluate potential associations with patient characteristics such as age, gender, and transfusion history.
3. Methods
This retrospective observational study was conducted at Imam Reza Hospital, affiliated with Kermanshah University of Medical Sciences, over a 24-month period from April 2017 to March 2019. All ATRs reported to the hospital’s blood bank during the study period were reviewed. The study included all patients who received blood transfusions and experienced or were suspected to have experienced an ATR during this period. Patients were included if they had complete documentation of transfusion details and post-transfusion monitoring. Cases with incomplete data or transfusions outside hospital protocol were excluded. No age or sex restrictions were applied. The hospital adheres to transfusion protocols adapted from the CDSCO technical manual. Paramedical staff trained in transfusion medicine performed safety checks prior to transfusion, including verification of the ABO-Rh group of the patient and the blood unit, product category, unit integrity (absence of clots, discoloration, or leakage), and expiration date. Before transfusion, each patient underwent a general physical examination. Vital signs were monitored 30 minutes after the transfusion began and hourly thereafter until completion. Patients were observed for signs and symptoms of ATRs, such as itching, urticaria, chills, rigors, nausea, vomiting, and dyspnea. In the event of a suspected ATR, blood and urine samples were collected pre- and post-transfusion. A standardized TR report form was completed, documenting the date and time of transfusion, reaction onset, vital signs before and after transfusion, the volume transfused, and clinical features observed.
Vital signs were monitored using calibrated automated bedside monitors (Mindray VS-800 or equivalent), capable of recording blood pressure, heart rate, respiratory rate, and temperature. Clinical staff documented symptoms in real-time using standardized observation sheets. Any abnormalities were immediately reported to the transfusion team via the hospital's internal alert system. The remaining blood product bag with the attached transfusion set, along with the completed reaction form and patient samples, were submitted to the blood bank. A physician evaluated each case to confirm or rule out a TR. Clerical errors were checked by cross-verifying the patient's blood sample, request form, and blood bag. Visual inspection of returned units was conducted to assess for clots or discoloration. If there was a delay between issue and transfusion, the conditions of storage were reviewed. Post-reaction plasma was examined for hemolysis. In cases of suspected hemolytic TRs, laboratory investigations included direct antiglobulin test (DAT), plasma free hemoglobin, and visual hemolysis assessment. Urinalysis was performed to detect hemoglobinuria. Serum bilirubin and lactate dehydrogenase (LDH) levels were assessed when applicable. The ATRs were defined as those occurring within 24 hours of blood component administration, excluding reactions due to incorrect blood product transfusion. Reactions were categorized according to the American Association of Blood Banks (AABBs) criteria. Cases with non-specific symptoms were categorized accordingly. To minimize bias, the study included all ATR cases reported in the defined period using consistent diagnostic criteria and institutional protocols.
A total of 488 TRs were included. The study size was determined by including all eligible cases reported within the two-year period. Quantitative variables such as age and transfusion volume were summarized using means and standard deviations; categorical variables, including types of reactions and products involved, were presented as frequencies and percentages. One limitation of the methodology is the potential for underreporting or misclassification of adverse reactions due to reliance on manual documentation. Despite training, human error in charting symptoms or vital signs may have introduced bias. These limitations were partially mitigated by standardized reporting forms, mandatory cross-checking procedures, and the involvement of trained transfusion medicine physicians in the review process. Statistical analysis was performed using SPSS software version 16 (SPSS Inc., Chicago, IL, USA). Normality of quantitative variables was assessed using the Shapiro-Wilk test. For continuous variables not normally distributed, the Mann–Whitney U test was used. Subgroup analysis was conducted based on age, gender, and blood component type to identify potential risk factors. The chi-square test was used to assess associations between categorical variables, with P-values < 0.05 considered statistically significant.
4. Results
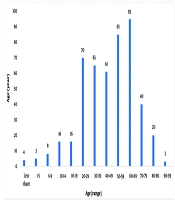
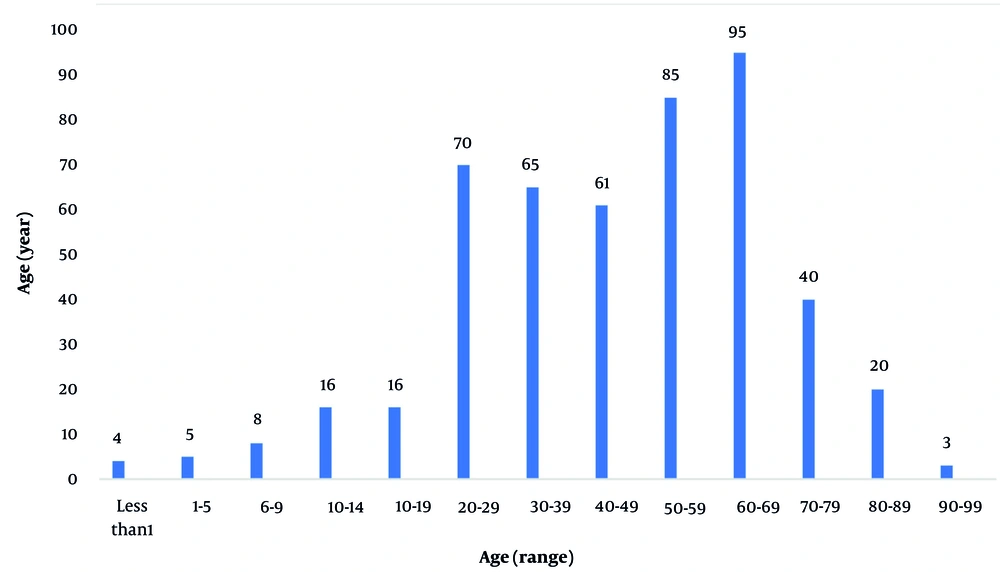
A total of 488 cases of TRs were identified over the 24-month study period. These reactions occurred in patients aged 1 to 99 years (mean = 49.1 ± 21.3 years) and included 229 males (46.92%) and 259 females (53.07%). The highest incidence of TRs occurred in patients aged 60 - 69 years (n = 95; 19.46%), followed by those aged 50 - 59 years (n = 85; 17.42%) (Figure 1).
The mean volume of transfused blood product at the time of the reaction was approximately 150 mL. Table 1 shows the distribution of reported reactions by hospital departments. The highest number of reactions occurred in the surgery ward (n = 173; 35.45%), followed by the emergency department (n = 88; 18.03%), internal medicine (14.34%), intensive care (11.47%), obstetrics and gynecology (9.43%), oncology (7.37%), and others.
| Departments | No. of Reactions |
|---|---|
| ICU | 38 (7.78) |
| Surgery | 173 (35.45) |
| Internal | 75 (9.22) |
| Emergency | 79 (16.18) |
| Women | 51 (10.45) |
| Room operating | 33 (6.76) |
| Oncology | 6 (1.22) |
| Dialysis | 13 (2.66) |
| Infectious | 11 (2.25) |
| Pediatric | 6 (1.229) |
| Post CCU | 2 (0.4) |
| Kidney transplant | 1 (0.2) |
a Values are expressed as No. (%).
The majority of reactions were associated with packed red blood cells (n = 358; 73.36%), followed by platelet concentrates (n = 86; 17.62%), fresh frozen plasma (n = 42; 8.6%), and cryoprecipitate (n = 2; 0.41%). The most common TR was an allergic reaction (n = 173; 35.45%), typically presenting with urticaria, rash, and pruritus. The second most common was FNHTR, reported in 164 cases (33.60%), characterized by a ≥ 1°C increase in body temperature from baseline. Less common reactions included hypotensive reactions (4.3%), anaphylactic reactions (2.5%), and TRALI, which occurred in 6 patients (1.22%) (Table 2).
| Types of Reaction | Packed Red Cells | Fresh Frozen Plasma | Platelet Concentrate | Cryo Precipitate | Total (%) |
|---|---|---|---|---|---|
| FNHTR | 108 | 6 | 33 | - | 169 (34.63) |
| TAD | 23 | - | - | - | 23 (4.71) |
| Allergic | 113 | 22 | 37 | 1 | 173 (35.45) |
| AHTR | 56 | 13 | 15 | 1 | 85 (17.41) |
| TACO | 24 | - | - | - | 2 (0.4) |
| TRALI | 4 | 1 | 1 | - | 6 (1.22) |
| Decrease blood pressure | 16 | - | - | 16 (3.27) | |
| Others | 14 | - | - | - | 14 (0.81) |
| Total | 358 (73.3) | 42 (8.6) | 86 (17.62) | 2 (0.4) | 488 |
Abbreviations: FNHTR, febrile non-hemolytic transfusion reaction; AHTR, acute hemolytic transfusion reaction; TRALI, transfusion-associated dyspnea.
a Values are expressed as No. (%).
No cases of AHTR or bacterial contamination were confirmed during the study period.
5. Discussion
This retrospective study identified 488 TRs over 24 months, with allergic reactions and FNHTRs being the most frequent. The highest incidence was observed in elective surgery patients, followed by emergency department patients. Packed red blood cells were the most common blood component involved. Age-wise distribution revealed that TRs were most prevalent in patients aged 60 - 69 years. This aligns with previous research indicating that elderly patients are at increased risk due to reduced physiological reserves and higher transfusion exposure (6). AHTRs, FNHTRs, anaphylactic responses, TRALI, and allergic reactions are acknowledged as major contributors to transfusion-related morbidity and death (7). The ATRs are immunological or nonimmune adverse responses that occur within 24 hours of receiving a blood transfusion. The estimated frequency of ATRs ranges from 0.2% to 10%, with a mortality rate of 1 in 250,000 (8). Recent studies have highlighted that surgical departments consistently exhibit the highest blood transfusion utilization, followed closely by emergency departments. These findings underscore the critical role of transfusions in managing patients in these high-acuity settings. In a comprehensive analysis conducted in South Korea, researchers observed that surgical units accounted for a significant portion of blood component usage. Specifically, in 2019, surgical departments utilized 9,462 units of blood components, which decreased to 5,728 units in 2020, reflecting a 39.5% reduction. Despite this decrease, surgical departments remained among the top consumers of blood products, second only to intensive care units. This study also noted that intraoperative transfusions constituted the majority of blood use within surgical departments (9).
Emergency departments also demonstrate substantial transfusion activity. A multicenter observational study in Spain revealed that only 54.9% of red blood cell transfusions in emergency settings were deemed appropriate based on clinical guidelines. This indicates a significant proportion of transfusions may be unnecessary, highlighting the need for stringent transfusion protocols in emergency care (10). Furthermore, a study examining blood utilization in emergency departments across five hospitals found that 41% of red blood cell units were unnecessarily transfused. The study emphasized the importance of reassessing transfusion practices to minimize overuse and ensure patient safety (11). Allergic reactions followed by FNHTR (33.60%) were the most prevalent symptoms (35.45%) in our investigation, and they were associated with a variety of skin manifestations such as urticaria, rashes, and pruritus. A study in North India reported FNHTR and allergic reactions were the most common of all types of adverse TRs (12). In contrast to our findings, Domen et al. observed a low rate of allergic TRs (17%) throughout a 9-year research period (13). The FNHTR was found to be higher in Nigerian hospitals, accounting for 65% of ATRs (14). In Serbia, 54.4% of reported TRs were febrile non-hemolytic responses, 38.3% were allergic reactions, and 1.11% were hemolytic reactions (14). The TRALI is an uncommon but significant cause of transfusion-related death. Mortality rates for TRALI vary, with estimates ranging from 5% to 25%, and potentially reaching up to 47% in critically ill or surgical patients (15). These figures underscore the severity of TRALI and the importance of preventive measures. It is an excellent mimicker of a wide range of clinical disorders and can be fatal. The TRALI occurred after a PRBC transfusion to a 14-year-old child with acute leukemia in our study (16). The donor sample, however, could not be tested for anti-HLA or anti-HNA antibodies, which could indicate vulnerable host factors. The TRALI can be reduced by carefully selecting donors (17). In this study, we registered 6 TRALI (1.22%) cases. From 1991 to 2002, Wallis et al. conducted observational research at the Freeman Hospital in the United Kingdom. The TRALI has been identified in eleven cases during the last 12 years (18). The authors of another study suggested that TRALI is more common than previously assumed, with an overall frequency of 1 case in 1120 cellular components transfused (19).
We discovered that red cell transfusion was the most prevalent cause of ATRs followed by platelet concentrates and FFP at 73.3%, 17.62%, and 8.6%, respectively. Our findings were consistent with those of Grujic et al., who found that erythrocytes were the most common cause of TRs, followed by fresh frozen plasma and platelets (20). Furthermore, another study found that red blood cell transfusions were responsible for almost half of the ATRs (but not severe reactions). On the other hand, FFP or platelet components were linked to two-thirds of the most severe reactions (13). The incidence of TACO in the current study was 2 cases of .... Praveen Kumar et al. and Popovsky reported in two different studies that the incidence of circulatory overload was estimated to be 1 in 380,658 blood transfusions and 1 in 3,168 (0.03%) patients transfused with PRBC (21, 22). Rapid transfusion of blood components should be avoided, and the AABB advises an infusion rate of 204 mL/min for RBCs and 'faster' rates for plasma and FFP (23). Patients with severe anemia (Hb 4 - 5 g/dL) are at higher risk of TACO because they are already in a hyperkinetic condition, with the heart being intolerant to even small increases in blood volume (24). The study is limited by its retrospective design and reliance on clinical reporting, which may lead to underreporting or misclassification of TRs. Laboratory confirmation was not available for all cases, and potential confounders such as pre-existing patient conditions were not controlled. Furthermore, being a single-center study limits the generalizability of the findings. The necessity of reporting all major and minor transfusion events to the transfusion service should be understood by all resident doctors and nurses on the ward, especially at night and in busy settings. Only by establishing a hemovigilance system can progress toward the aim of safe transfusion be made. There is severe concern about the underreporting of adverse reactions owing to clerical errors since it calls into doubt the technologist's expertise, efficiency, and service, as well as the administration's competence to administer the system. As a result, the obligation falls on the head of the transfusion system, who must be extremely watchful and investigate the core reason to fix the problem.
The implementation of robust hemovigilance systems — such as those advocated by the International Haemovigilance Network (IHN) — has been shown to reduce adverse transfusion events through real-time reporting, root-cause analysis, and training (25). Hospitals that adopted electronic hemovigilance tools demonstrated improved detection and more accurate classification of TRs, which in turn allowed for timely intervention and better patient outcomes (25). Our findings corroborate previous reports on the predominance of allergic and FNHTRs among TRs. However, the incidence rates should be interpreted cautiously due to the potential underreporting inherent in retrospective studies. Comparison with other regional and international data suggests variability in reaction rates possibly due to differences in hemovigilance systems, transfusion practices, and patient populations. Since this study was conducted at a single tertiary care center, the results may not be generalizable to other settings, especially those with different patient demographics, transfusion protocols, or hemovigilance systems. Multicenter prospective studies are recommended to better understand the incidence and spectrum of TRs.
5.1. Conclusions
The majority of the adverse reactions were found in elective surgery patients, followed by those in the emergency unit. The most frequently observed reactions were allergic, followed by FNHTRs. This may still represent an underestimation of the actual incidence due to underreporting — a challenge that can be mitigated through a robust hemovigilance system. To move toward safer transfusion practices, emphasis should be placed not only on adopting advanced technologies but also on optimizing existing systems. Practical steps include implementing electronic reporting platforms for real-time reaction monitoring, establishing standard operating procedures for transfusion across departments, and integrating hemovigilance metrics into hospital quality indicators. Moreover, hospitals should ensure the availability of adequately trained and dedicated transfusion staff, enforce mandatory reporting of all adverse transfusion events, and maintain an active hospital transfusion committee to oversee compliance and quality improvement. Regular continuing medical education (CME) programs tailored to both clinical and paramedical personnel can strengthen frontline recognition and response to TRs. At the policy level, integrating hemovigilance reporting into national health information systems and mandating regular audits at institutional and regional levels can reinforce accountability and promote systemic learning. These actions collectively can reduce transfusion-related adverse events and advance the goal of achieving near-zero-risk transfusion practices. Strengthening hemovigilance systems is critical for accurate detection, reporting, and prevention of TRs. Training of healthcare professionals and systematic monitoring are essential to improve transfusion safety.