1. Background
The experience of intraoperative awareness (IOA) and recalling events during general anesthesia (GA) has always been a concerning issue for both patients and anesthesiologists (1, 2). Despite significant progress in anesthesia methods, cases of IOA, although rare, still occur. Studies have mentioned nightmares, irritability, fear and anxiety, and changes in memory due to IOA (3, 4). The use of special complementary drugs can effectively increase the depth of anesthesia and decrease the probability of IOA. For example, opioids such as fentanyl or sufentanil are often used to induce analgesia and reduce patient recall (5, 6).
General anesthesia is not the first choice in CS unless medically necessary, such as in emergent situations (e.g., placental abruption, cord prolapse, antenatal placental bleeding, and fetal distress). The risk of surgical site infection, venous thromboembolism, difficult tracheal intubation, loss of airway control, anoxia, and aspiration of gastric contents is increased with GA (7). It should be noted that the incidence of accidental awareness during general anesthesia is relatively higher compared to other surgeries (8). A systematic review and meta-analysis recently pointed to the preventive role of benzodiazepines in IOA. However, such interventions are not permitted in CS (9). According to the known risk factors of IOA, including female gender, age, and the severity of perioperative anxiety, as well as restrictions on the prescription of premedications, CS is classified as a high-risk surgery for IOA (10). In a previous study, we found that the incidence of IOA in CS in our hospital was at the highest rate determined in the literature, indicating the need for an effective preventive intervention (11).
Studies have demonstrated that lidocaine can be used safely in CS with different routes of administration (12-17). In this study, for the first time, the topic of IOA in CS was examined from the perspective of solving the problem, and the efficacy and safety of prophylactic administration of intravenous lidocaine on IOA was investigated. Of course, the safety and benefits of lidocaine in CS have been shown in previous studies (13, 14, 18-22). However, the use of intravenous lidocaine in CS was limited to hemodynamic status, anti-inflammatory properties, and postoperative analgesia. None of the studies have addressed the effectiveness of lidocaine in reducing IOA. The idea was shaped according to valid evidence indicating that intravenous lidocaine can decrease BIS values and increase the depth of anesthesia (23).
Objectives:
2. Objectives
The aim of this study was to investigate the effect of a single dose of preoperative intravenous lidocaine on IOA in CS under GA.
3. Methods
This study was conducted at Alzahra Hospital, a referral center affiliated with Guilan University of Medical Sciences (GUMS), Guilan, Iran, from August 2023 to November 2024.
3.1. Inclusion Criteria
Women aged between 18 and 45 years, who were candidates for CS under GA (emergent or non-emergent), with American Society of Anesthesiology (ASA) physical status classifications I or II, and who were willing to participate.
3.2. Exclusion Criteria
Uncooperative women, those with difficulty in communication, drug abuse or psychological disorders, sensitivity to local anesthetics, and a history of IOA in previous surgeries were excluded. If intraoperatively, unexpected situations such as maternal unstable hemodynamics or the need for massive transfusion occurred, or if the mother was transferred to the ICU or the neonate to the NICU, she was excluded and replaced with another case. All enrolled women underwent the same anesthesia and surgery methods, and basic monitoring included non-invasive arterial pressure (NIBP), electrocardiogram (ECG), heart rate (HR), and pulse oximetry (SpO2). An anesthesia technician who was not engaged in this research performed the allocation using randomization quadruple blocks with a ratio of 1:1 for two groups: Lidocaine and placebo. In the lidocaine group, 1 mg/kg intravenous lidocaine was injected before induction of anesthesia, and in the placebo group, the same volume of normal saline was injected. The mentioned dose of lidocaine was chosen based on previous studies that confirmed its safety in CS (21, 24).
3.3. Anesthesia Management
First, preoxygenation was performed with a fitting face mask for 3 minutes. Then, propofol 2.5 mg/kg and succinylcholine 1 - 1.5 mg/kg were administered for rapid sequence induction, and tracheal intubation was performed. A tracheal tube size of 7 mm was chosen; however, smaller sizes of 6.5 mm and 6 mm were also available for unexpected situations. After that, 0.3 to 0.4 mg/kg of atracurium was injected, and anesthesia maintenance was performed with 0.5 - 0.75 minimum alveolar concentration (MAC) of isoflurane. After delivery, a short-acting benzodiazepine, midazolam 0.012 mg/kg, a short-acting opioid, fentanyl 2 µg/kg, and nitrous oxide 50 - 70% were added. At the end of the surgery, the residual neuromuscular block was antagonized by a mixture of 0.02 mg/kg atropine and 0.03 mg/kg neostigmine. Extubation was performed with the mother fully awake and airway reflexes maintained. After surgery, she was closely monitored.
The two hemodynamic parameters, including HR and MAP, were recorded at four time points: Before injection of lidocaine (T0), immediately after intubation (T1), at the end of surgery (T2), and after extubation (T3). When the mother was completely awake and cooperative in stable conditions and postoperative complications such as pain, shivering, nausea, and vomiting were controlled, a direct interview was performed. The assessment tool was a questionnaire obtained from Arefian and Fathi's study, containing women's characteristics (age, BMI, level of education, and history of anesthesia), gestational age, 1- and 5-minute Apgar scores, duration of surgery, and 14 items regarding the last memory before unconsciousness and the first recalled event after emergence from anesthesia, and 5 states of IOA (25) The Content Validity Index (CVI) and content validity ratio (CVR) of the mentioned questionnaire were confirmed by a previous study (11). In terms of IOA grading, grade 0 represents no recall and full unconsciousness, while grade 5 indicates full consciousness, intraoperative clear recall, emotional sequelae, distress, and pain (26).
3.4. Sample Size
Based on the study by Bazin et al. (27)and the estimation of a significant difference in BIS values between the lidocaine and placebo groups, assuming an effect size of 0.8, with a 5% error and 80% power, the sample size was calculated as 26 patients in each group using G*Power 3.1.9.7 software.
3.5. Statistical Analysis
SPSS software version 16 was used to analyze the data. Chi-square, Fisher’s exact, t-test, and repeated measures ANOVA tests were employed. Parametric data were described as mean ± SD, and nonparametric data were presented as median (range). A P-value less than 0.05 was considered statistically significant.
3.6. Ethics Committee Approval
The study protocol was approved by the Ethics of Research Committee of the University (IR.GUMS.REC.1402.311) and was registered in the Iranian Registry of Clinical Trials (IRCT) with the code IRCT20170314033069N6. Informed consent was obtained from the patients who agreed to participate.
4. Results
A total of 52 women with a mean age of 32.17 ± 6.48 years and a BMI of 28.69 ± 2.3 kg/m2 participated in the study. Maternal demographic data, number of gravidae, history of CS and anesthesia, 1st and 5th minute Apgar scores, surgery time, and CS status are presented in Table 1. No significant difference was observed in terms of the incidence of IOA between the two groups of lidocaine and placebo. A significant relationship was observed between BMI and IOA (P = 0.003), but not in terms of age (P = 0.062), level of education (P = 0.742), history of anesthesia (P = 0.257), 5-minute Apgar score (P = 0.967), and surgery time (P = 0.252).
| Status and Groups | Lidocaine | Placebo | Total | P-Value |
|---|---|---|---|---|
| Age (y) | 31.88 ± 6.39 | 32.46 ± 6.69 | 32.17 ± 6.48 | 0.752 |
| Education level | 0.391 | |||
| Illiterate | 1 (4) | 3 (12) | 4 (8) | |
| Elementary | 3 (12) | 6 (23) | 9 (17) | |
| Middle school and high school | 4 (15) | 6 (23) | 10 (19) | |
| Diploma | 12 (46) | 7 (27) | 19 (37) | |
| Academic educated | 6 (23) | 4 (15) | 10 (19) | |
| BMI (kg/m2) | 28.8 ± 2.49 | 28.58 ± 2.15 | 28.69 ± 2.3 | 0.74 |
| History of anesthesia | 0.375 | |||
| Yes | 7 (27) | 10 (39) | 17 (33) | |
| No | 19 (73) | 16 (62) | 35 (67) | |
| History of cesarean section | 0.579 | |||
| Yes | 12 (46) | 14 (54) | 26 (50) | |
| No | 14 (54) | 12 (46) | 26 (50) | |
| Gestational age (wk) | 36.96 ± 1.07 | 37.57 ± 1.27 | 37.26 ± 1.2 | 0.065 |
| Gravidity | 2 ± 1.16 | 2.07 ± 1.29 | 2.03 ± 1.22 | 0.823 |
| Surgery time (min) | 53.84 ± 5.34 | 51.92 ± 5.84 | 52.88 ± 5.63 | 0.222 |
| Apgar 1st (min) | 7.88 ± 0.99 | 7.65 ± 0.97 | 7.76 ± 0.98 | 0.402 |
| Apgar 5th (min) | 8.92 ± 0.68 | 8.84 ± 0.73 | 8.88 ± 0.7 | 0.698 |
| Cesarean status | 0.211 | |||
| Emergency | 5 (19) | 9 (35) | 14 (27) | |
| Elective | 21 (81) | 17 (65) | 38 (73) |
a Values are expressed as mean ± SD or No. (%).
Overall, a total of 8 women (15.3%) experienced IOA. Among them, 15 items were recorded. "Dreaming during surgery and anesthesia" was reported by 53.3%, and "feeling the manipulation of the surgical area" by 26.6% were the main reported items. Inability to move during anesthesia, hearing during anesthesia, and feeling pain during anesthesia were each reported by 1 (6.7%) mother and were the least detected items (Table 2).
| Awareness Status | No. (%) |
|---|---|
| Inability to move during anesthesia | 1 (6.7) |
| Hearing during anesthesia | 1 (6.7) |
| Dreaming during anesthesia | 8 (53.3) |
| Feeling pain during anesthesia | 1 (6.7) |
| Feeling the manipulation of the surgical area during anesthesia | 4 (26.6) |
Regarding the last remembered memory before anesthesia induction, 17.3% of women experienced unpleasant conditions, 4 women (7.7%) reported pain, and 5 (9.6%) experienced anxieties and fear of death. Putting on a face mask and being told to take a deep breath was the most common recalled event (48.1%). A total of 21.1% of women reported unacceptable conditions such as severe pain, suffocation, suctioning, and inability to move as the first remembered item immediately after emergence from anesthesia. Incomprehensible noise and crowds were the most frequently reported items after emergence from anesthesia (38.5%) (Table 3). The last recalled item and the first remembered item were also compared between the two groups (Table 4).
| Variables and the Recalled Event | No. (%) |
|---|---|
| The last memory before anesthesia | |
| Putting a face mask and saying take a deep breath | 25 (48.1) |
| Nurse and Anesthesiologist's general talks | 7 (13.5) |
| Prayer and supplication to God | 3 (5.8) |
| Pain | 4 (7.7) |
| Feeling of fear and anxiety about surgery, anesthesia, and death | 5 (9.6) |
| Washing and sterilizing the abdomen | 5 (9.6) |
| I do not remember anything during a smooth induction | 3 (5.8) |
| The first memory after emergence from anesthesia | |
| Hearing: Swallow your saliva and take a deep breath/your surgery is over/open your eyes | 10 (19.2) |
| Asking about my baby’s health and gender | 11 (21.2) |
| Severe pain | 4 (7.7) |
| Vague and incomprehensible noise and crowds around me | 20 (38.5) |
| Feeling suffocated, cold, or hot | 2 (3.8) |
| Suctioning and the presence of a tube in my mouth | 3 (5.8) |
| Inability to move | 2 (3.8) |
| Variables | Status | Lidocaine | Placebo |
|---|---|---|---|
| The last memory before unconsciousness | Putting a face mask and saying take a deep breath | 1 (50) | 12 (46.15) |
| Nurse and Anesthesiologist's general talks | 2 (7.69) | 5 (19.23) | |
| Prayer and supplication to God | 2 (7.69) | 1 (3.84) | |
| Pain | 2 (7.69) | 2 (7.69) | |
| Feeling of fear and anxiety about surgery, anesthesia, and death | 3 (11.53) | 2 (7.69) | |
| Washing and sterilizing the abdomen | 2 (7.69) | 3 (11.53) | |
| I do not remember anything during a smooth induction | 2 (7.69) | 1 (3.84) | |
| The first memory after emergence from anesthesia | Hearing: Swallow your saliva and take a deep breath/your surgery is over/open your eyes | 5 (19.23) | 5 (19.23) |
| Asking about my baby’s health and gender | 5 (19.23) | 6 (23.07) | |
| Severe pain | 2 (7.69) | 2 (7.69) | |
| Vague and incomprehensible noise and crowds around me | 10 (38.46) | 10 (38.46) | |
| Feeling suffocated, cold, or hot | 1 (3.84) | 1 (3.84) | |
| Suctioning and the presence of a tube in my mouth | 2 (7.69) | 1 (3.84) | |
| Inability to move | 1 (3.84) | 1 (3.84) |
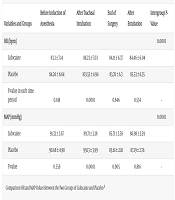
The trend of changes in hemodynamic parameters, including MAP and HR from T0 to T3, was significant in both groups (P < 0.0001); however, when comparing the two groups, a significant difference was detected only at T1 (P < 0.0001) (Table 5).
| Variables and Groups | Before Induction of Anesthesia | After Tracheal Intubation | End of Surgery | After Extubation | Intergroup P-Value |
|---|---|---|---|---|---|
| HR (bpm) | 0.0001 | ||||
| Lidocaine | 83.3 ± 7.14 | 88.23 ± 7.03 | 84.11 ± 6.37 | 84.46 ± 6.04 | |
| Placebo | 84.26 ± 6.64 | 103.53 ± 4.94 | 83.76 ± 6.3 | 85.53 ± 6.35 | |
| P-value in each time period | 0.618 | 0.0001 | 0.846 | 0.534 | - |
| MAP (mmHg) | 0.0001 | ||||
| Lidocaine | 91.33 ± 2.67 | 89.71 ± 3.38 | 85.71 ± 3.58 | 86.98 ± 3.59 | |
| Placebo | 90.68 ± 4.98 | 99.13 ± 3.99 | 85.61 ± 2.81 | 87.19 ± 2.76 | |
| P-value | 0.559 | 0.0001 | 0.905 | 0.816 | - |
a Values are expressed as mean ± SD.
5. Discussion
In this study, it was found that intravenous lidocaine administered prior to anesthesia induction could significantly maintain hemodynamic stability, which is justified by the drug's properties to blunt the sympathetic response. Studies have shown that endotracheal intubation (ETT) in CS, which is inevitably performed under light anesthesia, increases cortisol secretion and catecholamine release, systolic blood pressure by 50%, and heart rate by 20% from baseline, as well as increases the risk of IOA (28, 29). Regarding IOA, no significant difference was observed between the two groups of lidocaine and placebo, which may be due to insufficient lidocaine dosage, inappropriate timing, or the need for adjuncts like midazolam. Studies on IOA in CS are very limited, and the administration of lidocaine for this purpose has not been investigated.
In a study, BIS values were compared in sevoflurane amounts of 1% and 1.5%. The results showed that a 1.5% concentration kept BIS under 60 but was only effective until 10 minutes after delivery of the fetus (30). Abd El-Hamid et al. investigated the effects of intravenous dexmedetomidine 0.3 μg/kg/h with a low concentration of isoflurane on BIS values in 40 parturients undergoing CS. Women who received dexmedetomidine showed lower BIS values and more hemodynamic stability. It was concluded that the administration of dexmedetomidine with low-isoflurane concentration was effective in maintaining lower BIS values without adverse effects (31). Another study demonstrated that, according to frontal spectral EEG analysis, induction with propofol maintained a deeper depth of anesthesia compared to thiopental in pregnant women (32). Studies also reported that sevoflurane 1.5% with N2O for maintenance of GA in CS was sufficient for preventing IOA, while sevoflurane 1% may be inadequate for maintaining a proper depth of anesthesia (33) Khanjani et al. compared the incidence of IOA between two anesthetics, isoflurane and propofol, in elective CS. They found that among 90 women, three cases of IOA were confirmed in the isoflurane group (6.7%) and four cases in the propofol group (8.9%) (34).
Gottschalk et al. evaluated the effects of different doses of IV lidocaine: 0.5, 1.0, or 1.5 mg/kg on BIS values in the presence or absence of midazolam. There was no significant decrease in BIS values with the three sole lidocaine doses. However, in combination with midazolam, a significant decrease in BIS values was detected. They concluded that the effect of lidocaine on the depth of anesthesia was not direct and was effective in combination with midazolam (24).
IOA, as a multifactorial event, can be influenced by individuals’ genetic problems in drug metabolism or increased tolerance to anesthetic drugs, patient cooperation with the treatment team, and their level of knowledge. As such, it is essential that anesthesiologists communicate clearly with patients before surgery, carefully evaluate their medical history, and use a variety of safety protocols to reduce the likelihood of IOA. The type of surgery is also important in the case of IOA. Cardiac surgery, trauma patients, and CS are among the high-risk surgeries due to the restrictions on the administration of anesthetic drugs (35-37). Factors related to the skill of the anesthesiologist and the selection of correct techniques and appropriate drugs, as well as the use of necessary monitoring, are important. Sometimes, the dose of anesthetic drugs may not be sufficient, or there may be problems with the transfer or metabolism of the drugs, so sometimes things do not go according to plan. Furthermore, it is important to consider the potential impact of human medical error, such as in cases of incorrect drug administration (38-42). Anesthesiologists strive to achieve a delicate balance between ensuring adequate depth of anesthesia and avoiding excessive sedation. However, factors such as the patient's metabolism, drug interactions, and equipment malfunctions can lead to miscalculations or delivery errors. An inadequate dose of anesthetic drugs may result in momentary consciousness, insufficient amnesia, or even a painful experience (43, 44). Considering the positive association between anxiety and IOA, patient-doctor communication is vital to reduce patients’ perioperative anxiety and consequently the occurrence of IOA. Physicians and healthcare staff can effectively manage patient anxiety by creating a safe environment where patients feel comfortable expressing their concerns (45-47).
In addition, it is important to recognize that education plays a fundamental role in preparing patients for the surgical and anesthetic process. By receiving comprehensive preoperative instructions, patients can increase their knowledge and understanding of anesthesia and surgery methods, which ultimately leads to improved overall outcomes (48). The patient's physiological reaction to anesthetic agents may also influence the occurrence of IOA. Moreover, the patients’ age, medical history, and health status are contributing factors. Patients who chronically use painkillers may need higher doses of anesthesia to achieve the desired effect. Insufficient attention to individual patients’ differences can increase the risk of awakening during the operation. Finally, operating room conditions and equipment can be influential factors. Noisy operating rooms or sub-standard anesthetic drug delivery systems can lead to insufficient levels of anesthesia and IOA (10, 49, 50).
There are studies that support the distinction between intraoperative dreaming and IOA. Their results show that psychological complications occur following IOA. It was demonstrated that these complications were reported in 49.33% of patients who experienced awakening during surgery (4). Khanjani et al. reported IOA rates of 6.7% (isoflurane) and 8.9% (propofol), with no significant difference between the two agents. This suggests that both anesthetics carry a comparable risk of IOA in cesarean sections (34). Our study observed a higher overall IOA rate (15.3%), despite the use of lidocaine as an adjunct. This discrepancy may stem from differences in anesthesia protocols (e.g., avoidance of benzodiazepines in cesarean delivery) or patient demographics. Notably, Khanjani et al. focused on primary anesthetics, whereas our study evaluated lidocaine as a supplemental agent, which may explain the divergent outcomes (34).
Abd El-Hamid et al. reported that dexmedetomidine, combined with low-dose isoflurane, significantly reduced Bispectral Index (BIS) values and improved hemodynamic stability, indicating enhanced depth of anesthesia and lower IOA risk (31). While lidocaine also stabilized hemodynamics (e.g., attenuated intubation-induced tachycardia), it did not significantly reduce IOA incidence. This contrasts with dexmedetomidine’s efficacy, likely due to their distinct mechanisms: Dexmedetomidine (an α2-agonist) directly augments hypnotic effects, whereas lidocaine’s primary action is peripheral anti-nociception.
Khanjani et al. studied awareness and Apgar scores in elective cesarean sections under general anesthesia with propofol or isoflurane (34). Abd El-Hamid et al. examined the effects of dexmedetomidine on BIS during cesarean sections under low-dose isoflurane (31). Gottschalk et al. found that systemic lidocaine decreases BIS in the presence of midazolam (24).
5.1. Limitations
Despite the fact that studies have mentioned that the patient’s expression of IOA and recalling of intraoperative events are the main indicators, the lack of BIS monitoring or electroencephalogram (EEG) analysis could be considered a limitation of this research.
5.2. Conclusions
This study showed that a single dose of intravenous lidocaine before induction of anesthesia could be used safely in CS and effectively reduced intubation irritation. However, the effectiveness of lidocaine in IOA prevention at this dose remains unproven. Therefore, to obtain practical results, further trials should address the proper dosage and timing of lidocaine administration in CS.