1. Background
Chronic hyperglycemia in diabetes is associated with damage, disruption and prolonged dislocation of various organs, especially the eye, kidneys, nerves, and cardiovascular system (1). Diabetes is a major public health issue due to its high prevalence and increased mortality rates. Cardiovascular disease is the leading cause of death in diabetic patients (2, 3). Researchers report that the risk of heart failure in diabetic men is twice and in diabetic women is 5 times higher than non-diabetic subjects (3). Empirical and clinical evidence suggests that diabetics are susceptible to cardiac muscle damage and independent of large and small vascular disorders (3). Such diabetes-induced heart muscle damage is recognized by early disorder of diastolic function with hypertrophy, apoptosis, and fibrosis of the heart muscle (4, 5). Myocardial fibrosis (1972) has been confirmed as a major product of the side effects of diabetes mellitus on the heart by Rubler et al. (6) and in both human histology and experimental studies (7). Some interventional biomarkers have clinical interest in collagen synthesis and degradation in the diagnosis and identification of cardiac fibrosis, one of the most important of which is galectin-3 (8, 9), a member of the family of Galectin, which contains 250 amino acids and has a molecular weight of 29 to 36 kilo Daltons (9). The exact location of galectin-3 in myocardium has not been determined yet; however, studies in experimental rats have shown that galectin-3 binding sites are predominantly found in the myocardium matrix, fibroblasts, and macrophages (10, 11). A bulk of studies have shown that galectin-3 can be a factor in the development of cardiac fibrosis (10, 11). In animal models, the expression of high levels of galectin-3 has been observed in heart fibrosis (11). There are many ways to diagnose heart failure. In addition to echocardiography, one of the most recent diagnostic factors in this disease is the level of galectin-3. The concentration of galectin-3 in healthy heart is low and its expression in heart failure increases (12), because galectin-3 can cause cell death and fibrosis. Hence, the increased level of galectin-3 in heart failure is important and of great value. Furthermore, it has been shown that increased levels of galectin play a role in the process of cancer, inflammation, and tissue damage (12). As galectin can regulate cell death in both intracellular and extracellular states, they are privileged and unique (13). Therefore, galectin-3 has been introduced as a new indicator for the diagnosis of heart failure, cancer, inflammation, fibrosis, and tissue damage (12). It has been reported that galectin-3 is significantly associated with the risk of death from acute and chronic heart disease (14). Although exercise is a major factor in the prevention of cardiovascular disease, the relationship between exercise and changes in galectin-3 levels as yet unknown (15). In several studies in this regard, the effectiveness of sport activity in reducing galectin-3 in cardiac patients has been investigated. For example, in a recent study by Billebeau et al. (16), 40 endurance training sessions have been shown to reduce galectin-3 plasma levels in cardiac patients. As a new indicator of heart failure, the role of galectin-3 has been less studied and, moreover, the effectiveness of exercise training in modulating this factor in cardiac patients is unknown.
A study showed that the activation of the protein kinase C (PKC) pathway increases the expression of galectin-3 and also inhibits galectin-3 inhibitory production of collagen-stimulated PKC (17). It has been reported that overexpression of PKC found in the myocardium is responsible for the development of cardiac hypertrophy, left ventricular fibrosis (18). PKC activity has increased in the heart of diabetes and its level is associated with ROS and PARP activity (19). Rising glucose levels tend to increase the activation of diacylglycerol (DAG) and activate the pathway of PKC from vascular tissues of diabetic patients, which has a major change in diabetic cardiomyopathy, including decreasing blood flow of tissues, increasing the extracellular matrix deposition, thickening capillary membrane and increased vascular permeability (20). It was shown in the study that PKC induces heart disease and heart failure through the expression of galectin-3 (17). Among the important and non-pharmacological approaches of researchers and specialists to maintain and promote cardiovascular health is the implementation of exercises training (21). There exists a dearth of research measuring these two factors in diabetic patients and the impact of exercise on them.
2. Objectives
In this study, we strive to study the effect of high intensity aerobic exercise on galectin-3 and protein kinase C in diabetic rats.
3. Methods
To do this research, after obtaining permission from the Research Deputy of Razi University of Kermanshah, with ethics code of 396-2-025, 30 Wistar male rats (weighing 140 ± 20 g) were randomly selected and were kept in Mazandaran-Babolsar University laboratory for 2 weeks and became familiar with the laboratory conditions. The rats were randomly divided into 3 groups (control (n = 10), diabetic control (n = 10) and exercises diabetes (n = 10). The rats were kept under controlled conditions of 12 hours of light and 12 hours of darkness (beginning of light was as 6 AM and for darkness was as 6 PM) as 5-rat groups in transparent polycarbonate shelves in an environment of 20 to 24°C, and moisture content of 45 to 55 percent with free access to standard water and food. Intraperitoneal injection of streptozotocin (STZ) solution in citrate buffer (PH = 4.5, 0.1 mol concentration) and 55 mg/kg of body weight was used to induce diabetic by intraperitoneal injection. At 14 days after injection, blood glucose concentration was measured by blood samples collected from the animal's tail using a glucometer. The criterion for diabetes was the blood glucose concentration greater than 250 mg/dL (22).
3.1. Research Protocol
The subjects performed 5 sessions of walking and running at speeds of 5 to 8 m/min and a zero-gradient slope for 5 to 10 weeks before performing the exercise protocol for two weeks. The training program for the training group was running on an ungraded treadmill specific to rodents during which the training lasted 60 minutes at a speed of 34 m/min and 5 sessions per week for 8 weeks (23, 24). To warm up, the subjects ran at 7 m/min for 3 minutes at the beginning of each training session and then to adjust to the desired speed, the speed of the treadmill increased 2 m/min. To cool the body at the end of each training session, the speed of the treadmill was reduced inversely so as to reach the initial speed. The entire training program was conducted on an ungraded treadmill made in Mazandaran University, Iran.
3.2. Biopsy From the Rats and Measurements
After 8 weeks of training, 48 hours after the last training session, the rats were first anesthetized by intraperitoneal injection of ketamine 5 units and Xylazine 2 units, then their chests were split and the hearts were removed. Galectin-3 levels were measured by ELISA kit, Elabscience American Manufacturing Co., with the following catalog number: E-EL-R0399 and using quantitative sandwich method (range: 2000 - 25 ppm/31 pg/mL and sensitivity of < 18.75 pg/mL). The values of PKC were measured by ELISA kit, Eastbiopharm Manufacturing Company of China, with the catalog number: CK-E91111 and using quantitative sandwich method (range of 150 ng/mL and sensitivity of < 0.22 ng/mL).
3.3. Statistical Methods
The collected data were analyzed running SPSS software version 21 and Shapiro-Wilk tests to show the normal distribution of data and one-way analysis of variance and Tukey’s post hoc tests. In these surveys, the significance level was considered to be less than and equal to 0.05.
4. Results
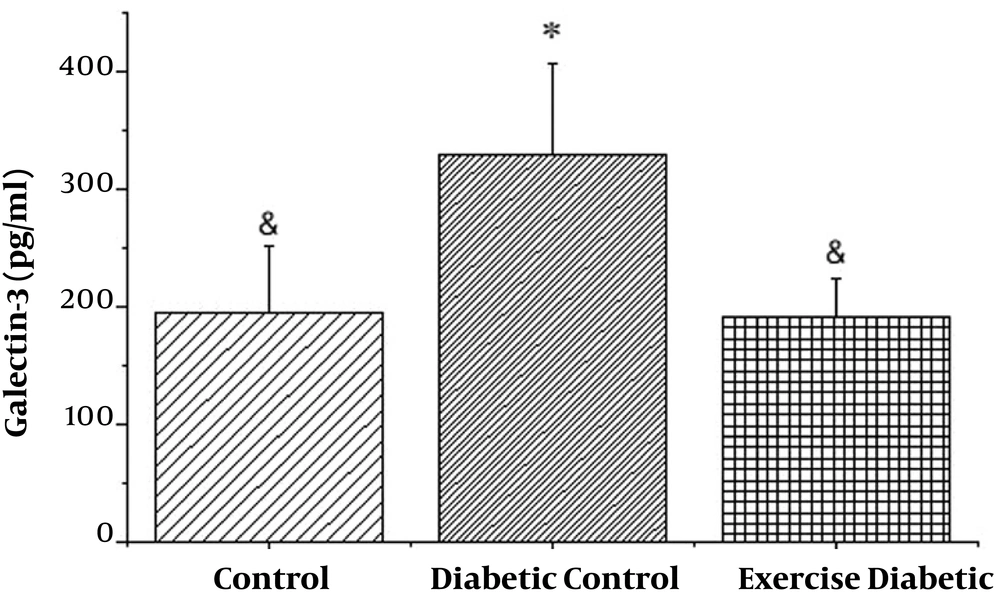
As shown in Figure 1, the levels of galectin-3 in the diabetic group were significantly higher than those in the control group (P < 0.001). Also, in the exercise diabetic group, in comparison to the diabetic group, the levels significantly decreased (P < 0.001). Therefore, 8 weeks of high intensity training had a significant effect on galectin-3 levels in diabetic rats (see Table 1).
| Group | Average | Standard Deviation | Standard Error | Minimum | Maximum |
|---|---|---|---|---|---|
| Control | 194.70 | 57.02 | 18.03 | 104.83 | 322.58 |
| Diabetic control | 329.06 | 77.44 | 30.81 | 169.15 | 441.43 |
| Exercise control | 191.76 | 32.86 | 10.39 | 134.75 | 247.99 |
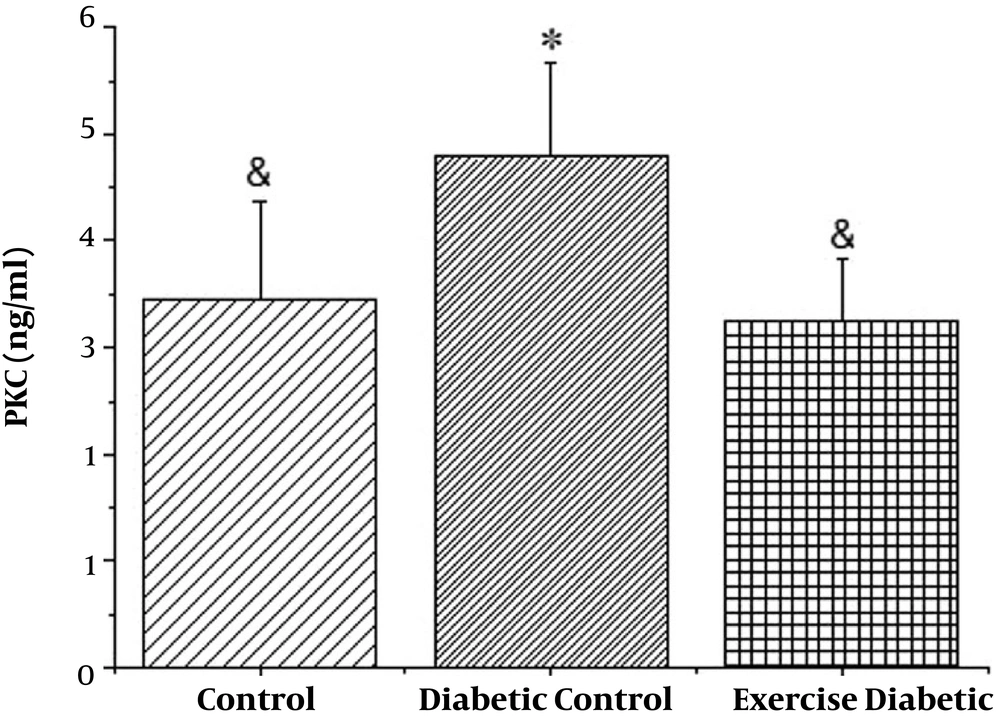
It is also seen in Figure 2 that PKC levels in the diabetic group increased significantly (P < 0.001), in addition to the results of the two diabetic groups with high intensity of exercise and diabetics, PKC levels decreased significantly in the training group (P < 0.001), resulting in 8 weeks high intensity training, which reduced the levels of PKC in diabetic rats (see Table 2).
| Group | Average | Standard Deviation | Standard Error | Minimum | Maximum |
|---|---|---|---|---|---|
| Control | 3.444 | 0.933 | 0.295 | 2.14 | 4.89 |
| Diabetic control | 4.786 | 0.883 | 0.279 | 3.43 | 6.20 |
| Exercise control | 3.249 | 0.572 | 0.181 | 2.28 | 4.10 |
5. Discussion
The results of this study showed that diabetes increased the levels of galectin-3 and PKC in diabetic rats compared to the control group. Also, the findings indicated that high-intensity endurance training reduced the galectin-3 and PKC levels in the diabetic exercise group compared to the diabetic control group. In patients with diabetes, cardiovascular complications are the main cause of illness and death and justify more than 65% of diabetic mortality. 33% of insulin-dependent diabetic patients have been reported to die until the age of 50 due to cardiovascular disease (25). Cardiac fibrosis is associated with an increase in the abnormal thickness of the heart cavity due to the excessive proliferation of cardiac fibroblasts and collagen deposition. Such increase in thickness reduces elasticity and ultimately reduces systolic and diastolic function of the heart. Various studies have shown that galectin-3 can be a factor in the development of cardiac fibrosis (11, 26). In experiments performed on rat samples, researchers observed that galectin-3 injections to the pericardial heart of healthy rats compared with those receiving placebo reduced EF, increased heart rate, and increased collagen. This experiment was conducted to prove the association of fibrosis with galectin-3 and finally concluded that galectin-3 had direct effects on the development of myocardial fibrosis and the progression of heart failure (27). Fibrosis and formation of scar are one of the major processes in abnormal changes in the heart, and are the main cells in the development and progression of scar tissue, fibroblasts, myofibroblasts and macrophages (28). In animal models, the expression of high levels of galectin-3 has been shown in liver fibrosis (29), kidney fibrosis (30), and heart fibrosis (26). Several studies have shown that galectin-3 is in various parts of the fibrosis in the change of heart condition (28). So far, limited studies have been conducted on the effects of exercise and its association with galectin-3 in healthy subjects and athletes. Although endurance exercise is a major contributor to cardiovascular disease, the relationship between exercise and plasma levels of galectin-3 is still unknown. Hattasch et al. (15) found that a 30-km-long endurance run in non-professional healthy men led to an increase in galectin-3; however, this was not related to cardiac function or other biomarkers or myocardial fibrosis. In line with these results, Salvagno et al. (31) examined the effects of a 60-kilometer marathon on 18 ultra-marathon trained athletes and concluded that levels of troponin I, galectin-3, and natriuretic pituitary levels of BNP increased significantly. In addition, the type of exercise can also affect the amount of galectin-3. So far as the researchers in this study have searched, only a few studies have been done on the effect of exercise on the level of galectin-3 in cardiac patients, but no study has been done on the effects of training on galectin-3 levels in diabetic patients. The study by Billebeau et al. (16) reviewed the effects of rehabilitation program on cardiac plasma biomarkers in chronic heart failure patients. Rehabilitation program included various endurance training of bikes and treadmills. The results of the study showed that plasma levels of galectin-3 factors decreased after cardiac rehabilitation program. Their final conclusion was that following a cardiac rehabilitation program in patients with chronic heart failure, general improvement in the neural hormonal profile was achieved (16). Their final conclusion was that following the program of cardiac rehabilitation in patients with chronic heart failure, general improvement in the profile of the hormones of the nervous system is proclaimed (16). In this study, the cardiac rehabilitation program has been the first treatment intervention to date, along with a reduction in galectin-3 plasma levels. Since galectin-3 has not been shown to affect medications or cardio coordinator devices, it is likely that this reduction in galectin -3 is directly related to exercise training (16). In sum, different cardiac biomarkers such as BNP and cardioprotective troponin are used to diagnose and predict patients with heart attacks or chronic heart failure, but recently newer biomarkers, which are a better indicator of pathophysiologic changes in deficiencies failures have been introduced. As mentioned above, one of these new indicators is galectin-3 involved in inflammation and cardiac fibrosis and is strongly associated with the development, severity and prediction of heart failure. Research has also shown that the expression of galectin-3 affects many of the processes involved in cardiac anomalies, including proliferation of myofibroblasts, tissue repair, inflammation, and ventricular changes (32). Increased levels of galectin-3 are significantly associated with the risk of death due to acute anomalies of the heart and chronic heart disease abnormalities (14). In addition, galectin-3 has been approved by the American College of Cardiology as a new indicator for the diagnosis of heart failure (11). PKC leads to the phosphorylation of a number of proteins that directly disturb the contraction of the heart and thereby disrupt the calcium levels in the heart. PKC activity increases in the heart of diabetes and its level is associated with ROS and PARP activity (19). Certain studies have shown that overexpression of PKC found in the myocardium is responsible for the development of cardiac hypertrophy and left ventricular fibrosis (18). A study showed that PKC pathway activation increases the expression of galectin-3, and inhibition of galectin-3 inhibits the production of collagen stimulated by PKC. It was shown in this study that PKC induces heart disease and heart failure through the expression of galectin-3 (17). Several animal and human studies have shown that activating PKC or enhancing PKC expression is associated with heart failure and PKC inhibition play a protective role for the heart. In a study, PKC reported an increase in regulation of galectin-3, which is a major contributor to regeneration, fibrosis and heart failure. Therefore, the increase in PKC leads to an increase in galectin-3, which increases fibrosis and heart failure. It was also found that Ang II, a known stimulant in hypertrophy and heart failure, could be activated from PKC and galectin-3 (17).
5.1. Conclusions
The results obtained from this study showed that diabetes increases cardiac fibrosis factors including galectin-3 and PKC. Increasing galectin-3 levels can be attributed to increase PKC because high-intensity endurance exercises reduces them. Furthermore, the decrease in galectin-3 can be attributed to a decrease in PKC, which indicates a positive effect of exercise.