1. Background
Hypertension is a multifactorial disorder, and any factor causes increased blood volume circulation or reduction of the diameter of blood vessels or contraction of the smooth muscles of walls of vessels can elevate blood pressure (BP). In any case, the mechanisms that develop and control BP are very complex and often unknown (1).
Oxidative stress is one of the factors that plays an important role in development of hypertension, atherosclerosis, and heart attack (2). In normal condition, there is a balance between the antioxidant defense systems and the level of production of free radicals. Nevertheless, the results of animal and human studies suggest increased oxidation stress and diminished antioxidant defense under hypertension conditions (3). In this regard, nitric oxide which has been reported to play a significant role in regulation of BP, will be impaired due to the oxidative stress resulting from accumulation of free radicals in the course of its production (4). Nevertheless, other factors such as changes in myeloperoxidase (MPO) and asymmetric dimethyl arginine (ADMA) can also influence the nitric oxide values (5).
MPO is a heme-containing peroxidase expressed in cell immune mainly neutrophils and to a lesser degree in monocytes (6). It has been reported that MPO to be a local mediator of tissue damage and the resulting inflammation in various inflammatory diseases such as chronic cardiovascular diseases. MPO causes production of large amounts of oxidizing intermediates through catalyzing respiratory burst reaction with the help of hydrogen peroxide, causing oxidative damage to cells and tissues (4).
In the other hands, ADMA which is developed out of metabolism of arginine and is its byproduct is the competitive internal inhibitor of nitric oxide synthase enzyme (7). It is possible that dimethyl arginine with L-arginine would compete as the substrate of nitric oxide synthase, thereby enhancing oxidative stress. However, there is some study about relationship between the plasma ADMA and BP in those with hypertension (8), and elevated ADMA levels have been observed in hypertensive patients (9).
In any case, to control the indices that develop impairment in the function of vessels of those with BP, benefiting from physical activity as well as supplements that improve the antioxidant system seem to be essential. In this regard, some studies (2016) has reported that aerobic training causes diminished BP and oxidative stress in those with hypertension (10). Nevertheless, there are no clear results available about the effect of aerobic training on the activity of MPO enzyme and ADMA in hypertensive individuals. It should be noted that aerobic training is very common physical exercise training of low to high intensity that depends primarily on the aerobic energy-generating process (11). It comprises innumerable forms such as, running a long distance at a moderate pace is an aerobic exercise training. This form of exercise is inexpensive and simple for all the people.
However, antioxidant supplements such as vitamin C (ascorbic acid) seems to be important in controlling BP. Ascorbic acid protects cells against oxidative stress (12). In this regard, Nishikawa et al. reported a significant reduction in systolic blood pressure (SBP) in response to consuming vitamin C in hypertensive rats (13). Also, it has been reported that consumption of vitamin C during the swimming training for ten weeks, five days per week, and one hour per session in rats can decrease the lipid peroxidation and increase level of TAC, although it is ineffective on enzyme and non-enzyme antioxidants and muscle damage (14). In any case, it seems that exercise and vitamin C have useful effects on BP in those with hypertension. However, the mechanism of their effect has not been examined comprehensively.
2. Objectives
Accordingly, the aim of the present study was to investigate the effect of 10 weeks of aerobic training alongside vitamin C consumption on myeloperoxidase, asymmetric dimethyl arginine, systolic blood pressure, and diastolic blood pressure (DBP) of hypertensive men. We will answer this question whether consuming vitamin C supplements during aerobic training would have a synergistic effect on the indices controlling blood pressure in hypertensive men or not.
3. Methods
3.1. Subjects
The present study was semi experimental research, with pre-post design. 62 middle-aged men (40 - 45 years) with hypertension who had referred to the healthcare centers in Urmia city in 2017 were selected as purposive sampling. Then, 40 men who were eligible for the inclusion criteria were chosen through purposive sampling.
The inclusion criteria of the subjects were: not being athlete, no tobacco use, and having hypertension. Further, the diagnosis of other conditions including kidney, respiratory, digestive disorders, diabetes, and consuming blood pressure reducing drugs during the study was the exclusion criteria of the subjects. In the first session, advantages, and possible risks, as well as the aerobic training were explained completely to the subjects. They were also requested to avoid taking any kind of drug especially antihypertensive drugs during the each exercise training session (15). During the exercise training protocol, all subjects were monitored by a physician. In addition, we assured subjects that their personal information will be kept confidential by the researchers. Then, all subjects signed the written informed consent form and announced their readiness to participate in the exercise program voluntarily. After that, 40 participants were randomly assigned into four groups: aerobic training with vitamin C (ATV; N = 10), aerobic training with placebo group (ATP; N = 10), vitamin C group (VC; N = 10) and control group (Con; N = 10).
3.2. Anthropometric and Functional Measurements
Subject’s body mass and height were measured (Seca, Mod 220, Germany). Also, body mass index, was calculated by following formula: weight (kg)/height (m2) (16). SBP and DBP was measured twice with intervals of at least 10 minutes in a sitting position using a digital cuff blood pressure monitor (Easy Life, Mod KD-5917, China), where the mean value of these two measurements was considered as the BP of each subject. Descriptive characteristics of subjects before starting of training were present in the Table 1.
| Groups | Age (y) | Body Mass (kg) | Height (cm) | BMI (kg/m2) | VO2max (mL/kg.min) |
|---|---|---|---|---|---|
| ATV | 47.3 ± 3.6 | 88.6 ± 6.6 | 175.8 ± 4.8 | 28.7 ± 0.9 | 41.7 ± 3.2 |
| ATP | 49.2 ± 3.7 | 88.2 ± 5.5 | 177.6 ± 5.1 | 28 ± 0.7 | 42.1 ± 4.1 |
| VC | 45.6 ± 2.1 | 93.4 ± 7.6 | 180.7 ± 4.9 | 28.5 ± 1.4 | 42.6 ± 3 |
| Control | 42.1 ± 3.2 | 96.2 ± 7 | 179.7 ± 5.2 | 29.7 ± 1.2 | 40.9 ± 2.6 |
3.3. Aerobic Training
Progressive aerobic training was done three days per week for 10 weeks. In the first week, the training duration was 20 minutes, which was done with the intensity of 75% of the maximum heart rate (HR max). As the physical fitness subjects improved, intensity and duration of running also gradually increased, where intensity reached 85% HR max in the last week, and the duration of running reached almost 45 minutes (17). At the beginning of each session, the subjects performed 10 minutes of warm up, and at the end, they cooled down for five minutes. During exercises training session, the intensity and HR was controlled and monitor by a pulse meter (polar, China). All of exercise training was done in gym on the treadmill (techno gym, Italy).
3.4. Vitamin C Supplement and Controlling Diet
ATV and ATP subjects consumed 500 mg of vitamin C or placebo tablets (Shahrdaru, Iran) after breakfast (18). To prepare the placebo, vitamin C tablets have been embedded into capsules. Similarly, inside the same sized and same-colored capsules, sucrose was embedded and used as placebo. Note that consumption of supplement and placebo in the exercise groups was double-blind.
3.5. Blood Samples and Biochemical Analyses
The blood samples were collected at pretest and posttest phases. In the pretest, before beginning the exercise program and after 12 hours of fasting, 5 cc of blood sample was gathered at 8 - 9 a.m. in a sitting position, after 5 minutes of rest. Also; blood sampling was performed 48 hours of the last training session. The blood samples were immediately kept in a styrofoam containing dry ice at 4°C and then transferred to laboratory for biochemical measurement and analysis. To separate the serum, centrifugation of blood samples was performed at 3000 rpm for 20 minutes. The serum levels of ADMA and MPO were measured by human kits (Cusabio, Chian) through ELISA method by ELISA-reader device (Pishtaz Teb Co.) at the wavelength of 450 nm. The ADMA kit had a measurement range of 7.8 - 500 ng/mL and sensitivity of 1.95 ng/mL. In this regard, the MPO kit had a measurement range of 1.56 - 100 ng/mL and sensitivity of 0.39 ng/mL. Further, the factors of intra-assay and inter-assay changes of these kits were less than 10 and 12%, respectively.
3.6. Statistical Analysis
The normality of data distribution and equality of variances were tested by Kolmogorov-Smirnov and Levene tests, respectively. In order to examine the effect of exercise training, and interaction of exercise training with vitamin C supplementation during 10 weeks, repeated measures analysis of variance test was employed. In case of significance of intra group changes, paired t-test, and for significance of intergroup changes, Bonferroni post hoc test were employed. All data were analyzed by SPSS V. 23 at the significance level of P < 0.05.
4. Results
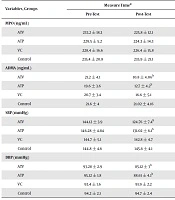
The results showed that the effect of time (effect of exercise training) is not significant about MPO (P > 0.05). Also, we found that the effect of group (effect of vitamin C supplementation) and interaction time-group (interaction exercise training and vitamin C supplementation) is not significant (P > 0.05). This means there is no significant between groups in MPO, and vitamin C supplementation was ineffective during exercise training on MPO (Table 2). Although, it was observed that ADMA diminished significantly to time (effect of exercise training) or training in the ATV (P = 0.001) and ATP (P = 0.001).
Also, the effect of group or vitamin C supplementation (P = 0.012) and interaction effect of exercise training with vitamin C supplementation in ADMA were significant (P = 0.001) (Table 2).
ADMA had decreased significantly in the ATV compared to VC (P = 0.031) and control (P = 0.001). Also, ATP showed a significant reduction only in comparison to the control (P = 0.001). ATV and ATP compared to the VC and control, SBP dropped significantly [(ATV vs. VC: P = 0.011; ATV vs. Control: P = 0.029), (ATP vs. VC: P = 0.039; ATP vs. Control: P = 0.044)]. Also, the ATV in comparison to VC (P = 0.017) and control (P = 0.047) and ATP in comparison to the control (P = 0.04) had only significant reduction in DBP. In other intergroup comparisons no significant difference was observed (Bonferroni post hoc test results).
The results also indicated that SBP and DBP in ATV and ATP diminished significantly, while in the VC and control, it did not change significantly (P > 0.05).
| Variables, Groups | Measure Timea | P Value | |||
|---|---|---|---|---|---|
| Pre-Test | Post-Test | Time | Group | Time × Group | |
| MPO (ng/mL) | 0.224 | 0.114 | 0.312 | ||
| ATV | 233.2 ± 18.3 | 225.8 ± 12.1 | |||
| ATP | 229.5 ± 5.2 | 224.3 ± 14.3 | |||
| VC | 228.4 ± 16.6 | 226.4 ± 15.8 | |||
| Control | 235.4 ± 20.8 | 235.9 ± 21.1 | |||
| ADMA (ng/mL) | 0.001 | 0.012 | 0.001 | ||
| ATV | 21.2 ± 4.1 | 10.8 ± 4.06b | |||
| ATP | 19.6 ± 3.6 | 12.7 ± 4.2b | |||
| VC | 20.7 ± 3.4 | 16.6 ± 5.1 | |||
| Control | 21.6 ± 4 | 21.02 ± 4.16 | |||
| SBP (mmHg) | 0.001 | 0.003 | 0.001 | ||
| ATV | 144.12 ± 3.9 | 124.76 ± 7.4b | |||
| ATP | 146.28 ± 4.84 | 131.02 ± 6.1b | |||
| VC | 144.7 ± 5.1 | 142.8 ± 4.7 | |||
| Control | 144.8 ± 4.6 | 145.6 ± 4.1 | |||
| DBP (mmHg) | 0.0001 | 0.006 | 0.001 | ||
| ATV | 93.28 ± 2.9 | 85.12 ± 3b | |||
| ATP | 95.12 ± 1.8 | 88.61 ± 4.1b | |||
| VC | 93.4 ± 1.6 | 91.9 ± 2.2 | |||
| Control | 94.2 ± 2.1 | 94.7 ± 2.4 | |||
aValues are expressed as mean ± SD.
bSignificant difference from pre-test.
The results indicated that the VO2max increased from 41.7 ± 3.2 and 42.1 ± 4.1 in ATV (P = 0.024) and ATP (P = 0.038) to 46.1 ± 5.1 and 45.6 ± 5.4, respectively.
5. Discussion
The aim of the present research was to investigate the effect of vitamin C supplement during aerobic training on changes in myeloperoxidase enzyme, dimethyl arginine, and blood pressure in men with hypertensive syndrome.
The results indicated that the VO2max increased from 41.7 ± 3.2 and 42.1 ± 4.1 in ATV and ATP to 46.1 ± 5.1 and 45.6 ± 5.4, respectively. VO2max Changes as a physiological index represent positive effect of protocol training in the current study.
The results showed that aerobic training alongside vitamin C supplementation could not reduction MPO to the baseline. However, Tanahashi et al. presented contradictory results about the effect of exercises training on MPO changes (19). It seems that the exercise training variables are influential in obtaining contradictory results. The duration of exercises was 10 weeks and its intensity was also different from that of other mentioned studies.
Also, the subjects in the present study were had hypertension syndrome. It is possible that changes MPO to aerobic training can be different in hypertension subjects, because hypertension has a direct relationship with inflammation (20). Also, in the present study, vitamin C supplementation was also used, which in turn can be one of the important reasons of these discrepancies. In comparison to the pretest results, some reduction was observed in the posttest activity levels of MPO. Although the extent of reduction in the MPO in the subjects was not statistically significant, it is clinically valuable. In other words, aerobic training and vitamin C supplementation have been able to prevent production of MPO, and could even decrease its serum level to some extent.
The results of the present research also showed that the serum level of ADMA decreased significantly in hypertensive individuals after aerobic training with alongside vitamin C supplementation compared to the baseline. The findings are congruent with the results of Shabaaninia et al., and Serre et al., (18, 21). However, Tanahashi et al. reported that aerobic training could increase ADMA in postmenopausal women (22). Also, it has been reported that four weeks of running increased ADMA in rats in rats (23). The subjects in the study by Tanahashi et al. were postmenopausal women (22), In contrast, in the present study the samples were middle-aged men with hypertension.
The mechanisms of effectiveness of aerobic exercise on ADMA are still unknown. ADMA is affected by oxidative stress (24). Previous studies have shown that antioxidant treatment cause diminished concentration of ADMA in plasma (25, 26). Furthermore, aerobic training reduce the extent of oxidative stress (27). Therefore, it is probable that aerobic exercises cause reduced ADMA by reducing the extent of oxidative stress. Reduction of oxidation stress and expression of arginine methyl transferase protein and elevation of dimethyl arginine dimethyl amino hydrolase occurred during aerobic training.
It was also observed that SBP and DBP decreased significantly to aerobic training alongside vitamin C supplementation. It is probable that one of the effective factors in this trend has been reduced ADMA. This lead to elevation of nitric oxide production and eventually diminished blood pressure. In addition, in this research vitamin C supplementation was also used. As an antioxidant, ascorbic acid is influential for production of prostaglandins. Prostaglandins such as PG1-2 are made of unsaturated fatty acids especially linoleic acid, and is susceptible to spontaneous oxidation. PG1-2 are vasodilators, thereby reducing blood pressure. The effect of vitamin C on hypertension may be through affecting other nutrients such as sodium. Specifically, by reducing the sodium level of blood of patients with hypertension, vitamin C can exert its useful effect. This effect is due to reduction of norepinephrine released from the central part of adrenal glands, thereby resulting in decreased sodium level in the bloodstream (28). Furthermore, in addition to being exposed to hemodynamic forces resulting from bloodstream, blood vessels are also subject to muscular contractions and stretching resulting from contractions in response to physical activities, which are considered as extra vascular mechanical forces, which can affect vascular endothelial cells independent of the bloodstream. In this way, they can increase the sensitivity of endothelial cells to growth factors, thereby improving blood pressure (29).
Another probable mechanism of reduction of blood pressure is related to the nitric oxide (NO) produced by endurance training. Endurance training can lead to increase in the vascular shear stress. Shear stress is the tangential force of the flowing blood on the endothelial surface of the blood vessel (30). Endurance training increases blood flow in the tissues, including muscle tissue (31), which in turn imposes shear stress on the endothelial cells (32). The increase in shear stress results in an increase in the production of nitric oxide which in turn causes an increase in the guanosine monophosphate levels. This is followed by increased vasodilation and, finally, reduction of blood pressure (33).
However, in the present study we could not control nutrition and dietary regimen. It may be affect the results. Also. Subject in the present study consumed antihypertensive drug with different doses. Therefore, the result present study should considered with these limitations.
5.1. Conclusions
Generally, it can be concluded aerobic training plus vitamin C supplementation can result in a significant change in dimethyl arginine as well as systolic and diastolic blood pressure improved in men with hypertension. In any case, the extent of effectiveness of aerobic exercises with and without supplementation of vitamin C did not have a considerable difference in relation to the studied indices.