1. Background
Alzheimer’s is an age related disease and the chronic cause of dementia and degenerative brain especially in older adults’ brain (1-3). Today, Alzheimer’s is one of the most prevalent diseases of the nervous system (4). The two main factors in Alzheimer’s are the formation of senile plaques composed of amyloid beta peptide (Aβ) and neurofibrillary tangles (NFT) composed of hyper phosphorylated protein tau (5). Amyloid beta, a monomer and a highly hydrophilic peptide. It has 37 - 49 amino acids which are generated by amyloid protein proteolysis (APP) (6, 7). The accumulation and precipitation of Aβ1-42 as plaques in the brain is recognized as one of the main factors and primary phenomena in pathogenesis of Alzheimer’s disease (8, 9). Studies indicate that injecting Aβ into the hippocampus causes disorders in the learning ability and memory of rats and also leads to neurolysis and disorders in the performance of neurons (4).
Streptozotocin (STZ), as other N-nitrous compounds, is an alkylating agent that causes tissue damage (10). A single 1 - 3 mg/kg injection of STZ causes atrophy and destroys nerve cells (11). Bilateral injections of 3 mg/kg of STZ lead to cognitive deficit, pathological plaques and phosphorylation of Tau (12, 13). STZ reduces brain metabolism and acetylcholine release and this plays an important role in reducing cognitive performance in Alzheimer’s disease (14). Intracerebroventricular injection of STZ reduces metabolism by restraining the synthesis of adenosine triphosphate (ATP) and acetyl coenzyme A, consequently leading to cholinergic system impairment and reduction of acetyltransferase activity in the hippocampus; it enhances acetylcholinesterase activity in rat brain (14, 15). The increased expression of the tau protein in the hippocampus of ICV-STZ rat and some signs of Aβ accumulation in the meningeal capillaries were found, indicating that the likelihood of the onset of Alzheimer's disease in this experimental model, thus giving further support to the resemblance of this experimental model to human Alzheimer’s disease (16). Some studies showed that exercise is a suitable non-pharmacological method for reducing the risks of Alzheimer’s disease. They specified that exercise improves the memory and prevents its weakness in older adults (17).
Liu et al. showed that the number and size of Aβ plaques in the hippocampus of transgenic rat model with AD (APP/PSI) reduced significantly at the intensity of 45% - 55% VO2 max after 5 months of exercising on the treadmill. Also, Aβ1-42 levels reduced significantly after exercising on the treadmill. Therefore, they suggested that exercise has an inhibitory effect on Aβ levels (18). A study conducted by Yuede et al. showed that following 4 months of voluntary and forced running at low intensity (16 m/min) by transgenic rat model of AD (Tg2576), no significant differences were observed in Aβ levels in brain cortex and hippocampus in the control and exercise groups (19). Um et al. also showed that running on the treadmill for 16 months at low intensity led to significant reductions in Aβ1-42 protein levels in the brain of transgenic rat models of Alzheimer’s group (NSE/ APP saw) (20). Meanwhile, another study showed that a 3-week running exercise makes no changes in the levels of Aβ in hippocampus of transgenic rat models of Alzheimer’s disease (Tg2576) (21). Another study indicated that a 3-week running exercise at moderate intensity reduced soluble Aβ1-40 and soluble fibrillary Aβ in the cortex of Alzheimer’s disease model rats (22). Furthermore, Bo et al. suggested that running on a treadmill at an intensity of 45% - 55% VO2 max reduces Aβ in the hippocampus of transgenic rat model of Alzheimer’s disease (APP/PSI). They recommended that exercise can reduce Aβ levels by improving mitochondrial function in the hippocampus and this should be considered as a therapy method for Alzheimer’s disease (23).
However, endurance training is strongly recommended due its role in brain health. But one of the main reasons for not taking up such a training method is a lack of time in modern society (24). Creating a suitable, but shorter, exercise program with the characteristics of continuous endurance training is being considered by sports science experts. One of the training methods suggested by the experts of this field is high intensity interval training (HIIT), which is a powerful method for improving endurance performance with an advantage over traditional continuous endurance training in terms of saving time (25-27). Investigations have shown that doing these exercises for several weeks improves factors involved in metabolism such as maximum aerobic capacity, maximum activity of mitochondrial enzymes and mitochondrial biogenesis (28).
The question is asked whether HIIT can be as effective as traditional endurance training in keeping the brain healthy due to its shorter time period. Hence, here we used six weeks HIIT to survey amyloid beta 1-42 (Aβ1-42) levels in the Hippocampus of male Wistar rat models of Alzheimer's disease.
2. Methods
2.1. Animals
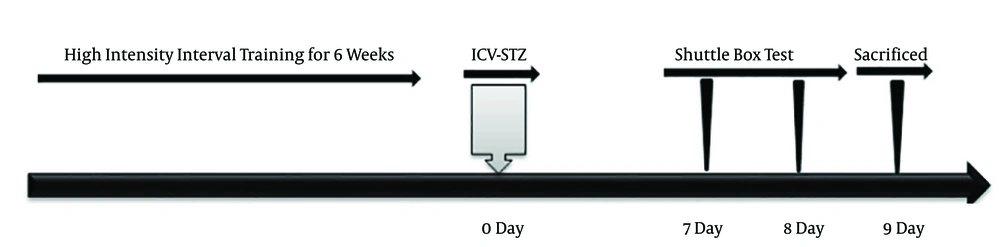
In this study, 35 twelve-weeks-old male Wistar rats were kept at temperatures of 22°C ± 2°C, light-dark cycle of 12:12 hours, and fed special feed for rats and water. All animals trained for 10 minutes a day for five days at speeds of 5 - 15 m/min to become familiar with the treadmill. Peak speed is calculated in order to determine maximum oxygen consumption using the Bedford et al. standard progressive test (29), which is standardized by Leandro et al. (30) for Wistar rats.
2.2. Experimental Design
Rats were randomly assigned into five groups (n = 7 per group): (1) control health, (2) control Alzheimer’s, (3) HIIT health, (4) HIIT Alzheimer’s and (5) sham. HIIT was performed 3 days a week, including warm up, the main training (interval reps) and cool down. Rats warmed up and cool down for 5 minutes on the treadmill at an intensity of 40% - 50% of peak speed. HIIT repetitions involved 2 minutes at an intensity of 80% - 110% maximum speed and low intensity interval repetitions included 2 minutes at an intensity of 30% - 40% maximum speed (Figure 1).
2.3. ICV Injection of STZ
The animals were anaesthetized with ketamine/xylazine (ratio of 6/60 mg/kg) i.p. and put on a stereotaxic (dual manipulator model 51600, USA). The skin was removed above the skull, and the coordinates for the lateral ventricles were measured using the Paxinos (31) atlas (anterioposterior -0.9 mm, lateral 5 mm, and dorsoventrally -3.2 mm). A burr hole was made in the skull with a hand drill. A 28-gauge Hamilton 10 µL syringe and piston attached to a microinjection unit was lowered manually through the hole into each lateral ventricle. The exercise and control groups were injected bilaterally with ICV-STZ (3 mg/kg, 5 µL). The sham group underwent the same surgical procedures, and the same STZ volume of saline was injected (32). After surgery, the rats were kept in a well-ventilated room at 25°C ± 2°C in individual cages and had access to food and water ad libitum until they recovered full consciousness and then were housed together with three animals per cage.
2.4. Passive Avoidance Learning Test
The equipment for the passive avoidance task consisted of a light-dark box with 2 compartments (18 × 18 × 25 cm). A steel bar which can give an electric shock was installed on the floor of the dark compartment. The wall connecting the two compartments could be opened and shut like a guillotine. After leaving each experimental animal in the back compartment for one minute, they were placed in the front compartment for 10 seconds to adapt. In order to perform the transition test, a rat was placed in the lit compartment with its back to the restricted opening; 10 seconds later, the guillotine door was raised allowing the rat to enter the dark area before closing. After 1 minute, the rat was returned to the cage. Training started 24 hours after the transition test rat was placed in the lit chamber with its back to the guillotine door; the door was raised 10 seconds later. When the rat entered the dark compartment, the door closed and an electrical shock (75 V, 1.2 mA, 50 Hz) was delivered for 3 seconds. Then, the rat was taken out of the dark compartment and placed in the cage. In order to evaluate memory, 90 minutes and 24 hours after the electric shock, the rat was placed in the brightly lit compartment while the door between the two chambers was left open and delay time was recorded for the first entrance into the dark chamber. The latency to enter the dark compartment was measured up to a maximum of 300 seconds.
2.5. Sampling
After 10 days of I.C.V STZ, rats were sacrificed and their brains were taken out to dissect the hippocampus. The dissected brain parts were homogenized in 10 mM phosphate buffer (PB, pH 7.0) containing 10 µL/mL protease inhibitor to get 5% w/v homogenate. Collected hippocampus samples were kept at -80°C for consequent measurements. In order to measure Aβ1-42 levels, first 50mg of hippocampus tissue was placed in a cold citrate-buffered saline. Then the tissue was homogenized for 10 minutes and centrifuged. The liquid was transferred into microtube. This solution was used to measure Aβ1-42 levels in the hippocampus tissue with ELISA kits from ZellBio GmbH (purchased from Padginteb Co, Tehran, Iran).
2.6. Statistical Analysis
Comprisions between groups were performed by One-way ANOVA followed by Tukey test. A value of P < 0.05 was considered to be significant. All statistical calculations were done using SPSS software version 22.
3. Results
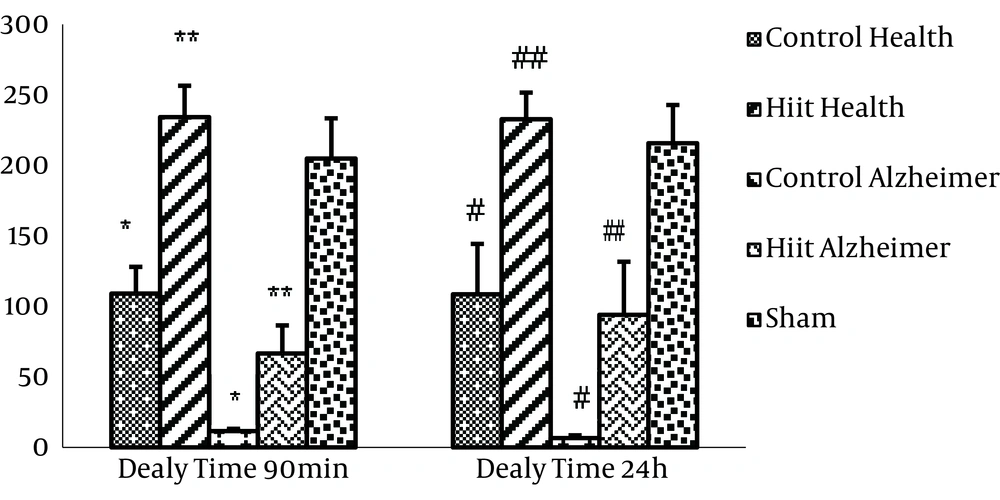
The results of ANOVA indicated that there was a significant difference among groups for delay times in entering the dark compartment (F4, 25 = 23.755, P = 0.001). Also, the Tukey post hoc test results showed that there were significant differences between the control health group and Alzheimer’s group, and the HIIT health group and Alzheimer’s group (P < 0.001) which indicates the validity of Alzheimer’s model in the related groups (Figure 2). There were no significant differences between the control health group and the sham group (P = 0.906). Therefore, the sham group was set aside when investigating other variables and the remaining groups were compared with the control group only.
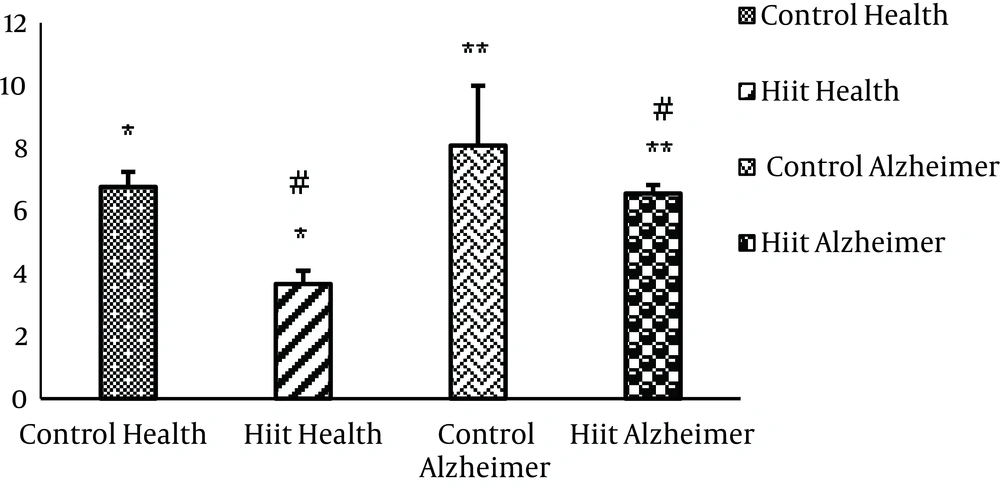
In Table 1, comparison of mean and standard deviation, as well as the findings of statistical test on the effects of HIIT on Aβ1-42 levels in groups are given.
| Group | Health Control | HIIT Health | Alzheimer’s Control | Alzheimer’s HIIT | F | P |
|---|---|---|---|---|---|---|
| Aβ1-42 (ng/g tissue) | 6.67 ± 1.18 | 3.66 ± 1.04 | 8.08 ± 0.46 | 6.55 ± 0.66 | 26.192 | 0.001 |
According to findings in Table 1, significant differences are observed in Aβ1-42 levels among research groups (F3,30 = 26.192, P = 0.001). Furthermore, the Tukey post hoc test results showed that Aβ1-42 levels were significantly higher in the control health group than in the HIIT group (P = 0.001) (Figure 3). Also, the Aβ1-42 value was significantly lower in the HIIT Alzheimer’s group than in the control Alzheimer’s group (P = 0.036). Aβ1-42 levels were higher in control Alzheimer’s than control health group but this difference was not statistically significant (P = 0.036).
4. Discussion
It seems that this is the first research in which the protective effects of HIIT on Aβ1-42 values in the hippocampus tissue of rat models of Alzheimer’s disease are investigated. Results demonstrated that the injection of 3 mg of I.C.V STZ leads to significant increases of Aβ1-42 in the hippocampus of rats (as the center of memory and learning). Changes in Aβ1-42 levels due to HIIT may help reduce the progress of the disease and protect the brain against Alzheimer’s. This disease is caused by the formation of aging plaques in the brain (33). Although this hypothesis has been reviewed in previous studies, Kang and Cho investigated the effects of a 6-week treadmill training (20 m/min) on insulin signaling and Aβ1-42 levels in rat models of Alzheimer’s by ICV-STZ injection. Their results showed significant Aβ1-42 reductions and insulin signaling enhancements in the brain of training Alzheimer’s rats compared to Alzheimer’s control group (6). Therefore, the results of their study agree with the results of this research.
In the study by Yuede et al. following 16 weeks of voluntary and forced wheel running on the treadmill (same as the voluntary group), no significant differences were observed in soluble Aβ levels (Aβ42, Aβ40) in the cortex and hippocampus tissue of the Tg2576 mouse model of Alzheimer’s disease in the control and training groups (19). The results of this study do not agree with the current research. The mechanism of changes made by training on Aβ1-42 are unknown, but the level of Aβ1-42 present in the brain is a balance between its production, cleaning and destruction.
Results show that regular exercise weakens neuronal apoptosis involved in Alzheimer’s disease pathogenesis by destroying or cleaning Aβ deposits. It seems that HIIT, like continuous aerobic training, is effective in reducing Aβ1-42 peptide in the hippocampus tissue of rats in Alzheimer’s induced by STZ. Since HIIT exercise is advantageous in terms of time and work volume, it seems that such training may be used as a non-pharmacological therapeutic method for Alzheimer’s disease in order to keep the nervous system healthy.