1. Background
Diabetes is one of the most prevalent diseases caused by metabolic disorders, which is considered as a major global challenge (1, 2). Type 2 diabetes involves a group of metabolic disorders that are characterized by hyperglycemia due to insufficient secretion of insulin, insulin resistance, or a combination of both (3). Evidence from clinical and experimental studies suggests that inflammation has an important role in the development of this problem (4). Because of the role of Adipose tissue as the source of inflammation, the relationship between obesity and type 2 diabetes, due to the production of cytokines is recent decades is more considered. The activation of adipokines triggers multiple responses. It repels insulin receptor after phosphorylation while at the same time adipokines activate other molecules that apply endocrine measures to different target tissues, including muscles, hypothalamus, and liver (5). Some adipokines, for example, the tumor necrosis factor-α (TNF-α) and IL-6 induces insulin resistance and inflammation (6), while other, for example, adiponectin and visfatin for glucose homeostasis and energy control is considered necessary (7, 8). According to the available research evidence, obesity is one of the health threats due to its association with metabolic and hormonal disorders such as lipid metabolism disorder, type two diabetes, cardiovascular disease and respiratory diseases (9). Based on the results of obesity studies and fat distribution in the body, especially in the central part (waist and abdomen), it is a strong predictor of metabolic disease. In fact, obesity can be described as a “new world syndrome”, which is considered to be the greatest health problem in the modern industrial and modern world (10). The prevalence of this disorder is increasing in all age groups in the world (10). Regular physical activity and optimal diet are factors that help prevent chronic illness by reducing fat levels, increasing insulin sensitivity, lowering blood pressure, and improving blood lipid profiles (11). In this regard, it seems that the introduction of well-designed training programs, favorable nutritional programs, and desired change in lifestyle can reduce the risk of developing vascular diseases (12, 13). However there are numerous finding about the effect of training on lipid metabolism in healthy subjects, the potential benefits of training on lipid metabolism in middle-aged people have been less widely considered. In this regard, studies have shown that the fitness program has a significant impact on the heath conditions of middle-aged people. This suggests that lipoprotein metabolism in aerobic training programs may increase.
Considering the role of obesity and its adipokaines in triggering some self-immune illness like type two diabetes and protective effect of training, some Studies have focused on the effect of physical activity and training on plasma lipokaines such as resistin, adiponectin and visfatine in human and animal samples. and in this area contradictory results have been reported. Among these, adiponectin and vidfatin are more investigated. Adiponectin is one of the most abundant hormone-like that secreted from adipose tissue, which, unlike others such as leptin and, resin, adipokines, is reduced in obesity (6). This hormone plays a major role in regulating the energy needed to maintain a homeostasis, fat and carbohydrate metabolism, and insulin sensitivity (6). Reported that aerobic training had no beneficial effects on serum adiponectin and resistin levels in obese women (14). On the other hand some studies reported that regular aerobic training, in addition to weight loss, body mass index, and fat percentage, reduced resin content and increased adiponectin in active subjects (15). The pivotal role of adiponectin in relation to the risk factors associated with metabolic syndrome, along with its anti-inflammatory effects, has been considered. Some studies have shown that reducing adiponectin concentration is associated with insulin resistance, hyperinsulinism, and hyperglycemia (15). In addition, various studies have reported an increase in adiponectin levels following weight loss and improved insulin resistance (16). According to the results, it seems that reducing adiponectin levels can be a major contributor to obesity and associated inflammatory disorders, including insulin resistance and type 2 diabetes. The results of studies indicate that increased concentration of plasma adiponectin leads to decreased insulin resistance, triglyceride, abdominal environment, abdominal/hip ratio, low-density lipoprotein, body fat mass, and high lipoprotein levels (15). Visfatine is one of the other important lipokine that its decrease such adiponectin has beneficial effects. It has been reported that visfatine has insulin-like properties (17) but it’s not clear that its acute and chronic changes how is affected by training and the results of study are limited and contradictory.
Today, special attention has been paid to various food additives. These compounds are important because they are herbal and are widely used in various dietary regimens. Cinnamon can be mentioned as herbs that seems to play an important role in the treatment and control of diabetes. It was reported in 1990 that cinnamon substances have insulin properties (18). One of the active ingredients derived from polymer cinnamon is hydroxyl methyl Kalkn, which acts like insulin (19). In type 2 diabetic patients, insulin receptor phosphorylation is reduced. On the other hand, cinnamon-soluble components inhibit insulin-receptor auto phosphorylation, a self-acting enzyme in the dephosphorylating of the receptor 2-insulin phosphatase (19). This itself increases insulin sensitivity. In some studies this has been shown that cinnamon can increase glucose catabolism by activating the insulin receptor and increasing glycogen synthesis (19).
Several studies have been carried out on the effects of various training methods (aerobic, resistance, concurrent) and its effective factors. Previous studies have found that intense training can increase the number of inflammatory cells, such as lymphocytes, monocytes, and neutrophils, all of which can release a wide range of cytokines and growth factors (20). Given the studies with contradictory results about the effect of physical training and training on blood adipokine, some studies have been done on the simultaneous assessment of aerobic and concurrent physical activity and diet modification. Considering that no research has been done on the effect of simultaneous use of cinnamon and aerobic and concurrent trainings on the level of adiponectin and visfatin serum level and the importance of using a better and more durable method to control the level of type 2 diabetes, which involves the use of cinnamon with aerobic and concurrent training, more research is required in this area.
2. Objectives
So the aim of this study was to measure the effect of aerobic and concurrent training with cinnamon on adiponectin and visfatin serum level in men with type 2 diabetes.
3. Methods
This is a experimental and applied study with pre and post-test, by four experimental groups, and one control group. The statistical population of the present study included overweight and type II diabetic men in Kermanshah who referred to a medical clinic. The statistical sample was 50 overweight men with type-2 diabetes mellitus voluntary participated in this research. The members of the samples were divided into experimental and control groups randomly. The subjects didn’t have any regular training experience in the past six mounts and with the control of investigators, avoid from any form of training except the study design training. Based on the clinical signs in the beginning of study, except of type II diabetes measured by fasting glucose (> 126), they were safe and in terms of body mass index (BMI)were in amplitude (> 25). In this study, initially the anthropometric indices of patients were measured so those abdominal and hip circumferences were measured at the most prominent point by an irreversible strip meter. The height of people without shoes and in a standing position was measured in such a way that the scapulars are tangent from the back with the wall. Weight and body fat percentage were recorded by using a body composition assay. The standing height and body weight were used to calculate body mass index. At the beginning of study, after completing the testimonial, questioner and physical activity level forms, subjects were randomly divided in five groups of 10 people. Five groups consist of aerobic training with placebo, aerobic training with cinnamon, concurrent with placebo, concurrent with cinnamon, and control group randomly. The cinnamon and placebo (strach) were consumed with subject in three 500 mg capsule per day while both had same shape, taste and smell. They have to follow their normal diet throughout study and avoid from any drugs and supplementation. Two day before the training course, the subjects after night rest and fasting state come to laboratory at 8 o’clock and 6 cc bloods was obtained from brachiocephalica vein. The samples were transferred under -4 to immediately to laboratory and with Wohan Chinese company hymen’s kit, by means of ELISA method measured.
While the experimental did their specific training protocols; the control group was banned from any form of training except normal daily activities. The training protocol for aerobic group was aerobic running by heart rate of 50 to 80 present of maximum heart rate (maximum heart rate = 220 -age). The training program was started by 20 minute and eventually was reached until 45 minute (21). Warm up and cooling down was performed for 5 minute at the beginning and the end of each session. The heart rate was controlled by polar barometer. the concurrent group did aerobic and resistance training alternately in each session. after 5 minute warm up, they first performed resistance training consist of two sets of twelve (2 × 12) by 70 percent of maximum strength calculated by the one maximum repetition equation (1 RM) 1 RM = weight (kg) × [1+ (0.033 × number of repetition)] for some selective multijoint resistance training. After resistance training, aerobic training consist of aerobic running was perfumed immediately by intensity of 50 to 70 percent of maximum heart rate. The total training time that begins from 20 minute and eventually reached to 45 minute was divided equally between two type of training e.g. aerobic and resistance training (22).
To analyze of data, in the descriptive analysis section, descriptive statistics indices such as mean and standard deviation were used and in inferential statistics, Shapiro-Wilk test was used to verify the natural distribution of data. The covariance and Bonferroni post hoc test was used to compare the groups. A significant level of 5% was considered in all calculations.
4. Results
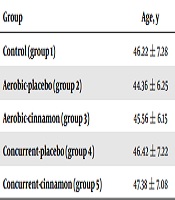
Table 1 showed the descriptive data.
| Group | Age, y | BMI, kg/m2 |
|---|---|---|
| Control (group 1) | 46.22 ± 7.28 | 28.85 ± 2.75 |
| Aerobic-placebo (group 2) | 44.36 ± 6.25 | 27.92 ± 2.65 |
| Aerobic-cinnamon (group 3) | 45.56 ± 6.15 | 29.65 ± 2.35 |
| Concurrent-placebo (group 4) | 46.42 ± 7.22 | 28.52 ± 2.46 |
| Concurrent-cinnamon (group 5) | 47.38 ± 7.08 | 27.84 ± 2.42 |
Age and Body Composition of Subject in the Beginning of Studya
Table 2 showed the normality of data by Shapiro-Wilk.
Tables 3 and 4 showed the change in research depended variables during pre to post-test are about the changing of the visfatin serum levels. The analysis of covariance and benferoni post hoc test showed there are significant difference between aerobic-cinnamon and aerobic-placebo with control group respectively (P = 0.056 and 0.001) but there is no significant difference between two experimental group (P = 0.088). In other world both groups similarly decrease the levels of visfatin. After 8 weeks of training.in concurrent training the finding showed both concurrent-cinnamon and concurrent placebo had significant difference with control group (P = 0.001) and there is no significant difference between two experimental groups and the decreasing of visfatin levels was same in two experimental groups (P = 0.006).
| Group | Pre-Test | Post-Test |
|---|---|---|
| Control (group 1) | 8.53 ± 0.619 | 8.514 ± 0.543 |
| Aerobic-placebo (group 2) | 8.61 ± 0.668 | 6.36 ± 0.465A |
| Aerobic-cinnamon (group 3) | 8.45 ± 0.688 | 6.29 ± 0.939A |
| Concurrent-placebo (group 4) | 9.241 ± 0.709 | 7.82 ± 0.803A |
| Concurrent-cinnamon (group 5) | 9.14 ± 0.592 | 6.53 ± 0.658A |
| Group | Pre-Test | Post-Test |
|---|---|---|
| Control (group 1) | 14.71 ± 0.415 | 14.75 ± 0.460 |
| Aerobic-placebo (group 2) | 14.51 ± 0.433 | 16.08 ± 0.445A |
| Aerobic-cinnamon (group 3) | 14.23 ± 0.365 | 15.35 ± 0.782A, B |
| Concurrent-placebo (group 4) | 14.45 ± 0.500 | 15.28 ± 0.815 |
| Concurrent-cinnamon (group 5) | 14.52 ± 0.421 | 16.567 ± 0.460 |
About the changing of the adiponectin serum levels, the analysis of covariance and benferoni post hoc test showed there are significant difference between aerobic-cinnamon and aerobic-placebo with control group respectively (P = 0.001 and 0.046) but there is no significant difference between two experimental group (P = 0.077). In other world both groups similarly increase the levels of adiponectin after 8 weeks of training.in concurrent training the analysis of variance and benferoi post hoc test showed both concurrent-cinnamon and concurrent placebo had significant difference with control group (P = 0.001) and there is significant difference between two experimental groups and the increase of adiponectin was more in concurrent group (P = 0.001).
5. Discussion
The main purpose of this study was to compare the effect of eight weeks of aerobic and concurrent (aerobic and resistance) training with supplementation of cinnamon on the levels of some adipokines (visfatin and adiponectin) in obese patients men with type 2 diabetes. The results showed that both groups of eight weeks of concurrent and aerobic training without cinnamon had a significant decrease in visfatin levels as well as an increase in adiponectin levels. Meanwhile, the use of cinnamon had a significant effect on the increase in adiponectin levels. However, the results showed that the concurrent training group with cinnamon had a more increase on the level of adiponectin in obese men with type 2 diabetes compared to the placebo-concurrent training group. The results of this study were consistent with the results of Kardiatun et al. (23) and Lee et al. (24). In addition, the results of this study were not consistent with the results of Nassis et al. (14). Probably the reasons for non-alignment with the results of these studies may be due to differences in training time, training intensity, and age of participants, supplementation of cinnamon and other environmental factors.
Currently, it is well known that the obesity especially central type is a one of the factor that related with insulin resistance and metabolic syndrome (9). Studies identified visfatin as an important adipokine, which is mainly produced in the fat tissue of humans. Visfatin may facilitate glucose control. The visfatin levels were related to age, WHR and fasting insulin, while after multiple regression analysis, it was found that only WHR had a positive correlation with plasma visfatin levels (25). Sedentary lifestyle are associated with the most constituents of metabolic disorders, and physical activity is correlated with the reduction of obesity risk, type 2 diabetes and cardiovascular disease (25). Therefore, changes in levels of adipokines may be a fundamental key to identify the desirable effects of the activity. Choi et al. (25) studied the simultaneous effect of concurrent aerobic and resistance programs on 48 plasma levels of non-diabetic women aged 50 - 55 years with overweight or obesity. Subjects were non-active who normally did less than 20 minutes of training during a week. The applied training program was 5 sessions of weekly training for 12 weeks, each session had 45 minutes of aerobic activity and 20 minutes of resistance activity. Subsequently, after 12 weeks of training, the subjects lost weight from 4 to 5 kg, the percentage of fat, waist circumference, fasting glucose and visfatin levels. Therefore, the combination of aerobic and resistance programs and weight loss significantly reduced plasma visfatin levels of overweight or obese women. Braun et al. (26) also showed plasma levels of visfatin in two groups of obese and type 2 diabetic patients aged 45 - 45 years before and after 12 weeks aerobic training. The training was performed at an intensity equal to 75% of maximum aerobic power, 4 sessions per week and each session for 1 hour. At the end of the training program, the waist size of subjects with diabetes was decreased, the maximum aerobic power of obese subjects was increased and visfatin levels decreased in both groups.
The results of this research regarding the significant change (inter-group) of adiponectin in subjects was inconsistent with the results of Sirico et al. (15), and is consistent with some other types of research like Reynolds et al. study (27). In Reynold’s research, 10 weeks of concurrent training resulted in a significant improvement in plasma adiponectin levels in inactive postmenopausal women. Abbenhardt et al. (28) examined the effect of diet and training on adiponectin in women, and observed that adiponectin was significantly increased and this index was directly related to insulin sensitivity. Some other studies suggest no significant changes in adiponectin, including, Reynolds et al. (27) evaluated the effect of weight loss (training and diet) on glucose, insulin, and adiponectin in women overweight and obese after menopause. They concluded that weight loss did not change the plasma concentration of adiponectin while plasma glucose and insulin were significantly decreased. In another study, 16 weeks of concurrent training did not have a significant effect on plasma leptin and adiponectin concentrations and body mass index (BMI) and weight of postmenopausal women. A number of recent studies have shown that training affects the concentration of adiponectin in plasma. However, most of these studies did not report significant changes in the concentration of adiponectin after training despite the difference in practice protocols (a period of training, intermittent and endurance trainings), and intensity of training. It has also been shown that diet and sports training increase adiponectin (29). In some studies, the effect of training on adiponectin and insulin sensitivity in elderly diabetic patients was observed and significant changes were observed in adiponectin concentration during training (endurance, strength, and combination). As the effects of low calorie diet and moderate (concurrent) activity on adiponectin were observed in a study in elderly subjects with insulin resistance. The results showed that the insulin sensitivity index improved in all subjects, but adiponectin increased significantly only among diabetic subjects. Moreover the effect of cinnamon supplementation is added to effect of training in this study. Cinnamon has insulin like effect that improves insulin senility and resistance. In concurrent group, the effect of training and cinnamon added to each other add led to more decrease in adiponectin level in compared with aerobic and cinnamon group and concurrent group. It seems the total consumed calories in concurrent group is more and adiponectin in more effected by total calories in compare with visfatin.
5.1. Conclusions
The results of this research showed that the effect of separation training on the consumption or non-use of cinnamon in aerobic and combination methods used in this study had a significant effect on measured parameters including adiponectin and visfatin, which was effective in improving the health of overweight men with type 2 diabetes. On the other hand, the simultaneous use of cinnamon supplementation with concurrent training compared to aerobic training was more effective in increasing the amount of adiponectin. Generally, the results of this study showed that the effect of aerobic and concurrent activity (endurance-resistance) and cinnamon extract together have a synergistic effect and increasing in adiponectin. Therefore, based on the findings of this research, it is suggested that combination of aerobic and the use of cinnamon can be a suitable approach to control blood glucose in type 2 diabetes.