1. Background
Iodine is an essential element for the production of thyroid hormones, and its deficiency is currently considered one of the causes of brain damage across the world. An adult needs 150 µg of iodine per day to grow normally, of which about 90% is gained from food and 10% from water (1-4). Iodine deficiency is one of the most important health issues in developing countries like Iran (5). Iodine deficiency disorders (IDDs) mainly affect the health of more than a third of the world’s population (6).
IDDs include goiter, cretinism, intellectual disability and physical disabilities such as growth retardation, movement and mobility deficiencies, strabismus, lack of muscular coordination and deaf-mute (7). Iodizing the salt is one of the effective ways to prevent and control IDDs (8, 9).
Storage conditions of iodized salt such as light, temperature, and humidity can affect the iodine stability. Previous studies have shown about 58.5% of iodine content was lost by storing salt at room temperature with a relative humidity of 30% - 45% in sealed bags after three years. Therefore, constant and accurate monitoring of iodine content in the produced and consumed salts in the community are very important (10). Similar studies indicate that iodine is reduced by physical environmental factors such as light, heat and humidity. Dasgupta et al. suggested that salt storage at high humidity significantly reduced iodine content (11).
In addition, Diosady et al. stated that high humidity led to the loss of iodine in iodized salt (12). Iodine less than the standard (30 ppm) in consumed salt causes failure in the country’s program against IDDs, while iodine more than the standard (50 ppm) has adverse effects on the community health (13).
2. Objectives
Considering the importance of iodine for community health, the present study evaluated iodine stability in different conditions of light, humidity and temperature in refined iodized salts.
3. Methods
In this descriptive-analytical study, samples of refined iodized salt marketed in Babol (5 salt brands labelled as 1, 2, 3, 4, and 5) were randomly selected and the initial content of iodine in the samples was measured. Then, iodine stability of samples was studied in different conditions of ambient light, darkness, humidity, without humidity and ambient and incubator temperatures of 37°C.
The samples were stored for three months under the specified conditions. The salt samples for ambient humidity were left open and those for non-humidity were stored sealed.
In order to measure the effect of light, sealed samples were exposed to ambient light while those for studying the effect of darkness were stored in a dark place. To monitor the effect of heat, the samples were stored at room temperature, in a refrigerator or an incubator of 37°C.
The iodine content of samples was tested every 15 days in the Chemistry Laboratory of Paramedical School in Babol University of Medical Sciences using titration method recommended by British Pharmacopoeia. The results were reported as mean ± SD after three repeats.
The results were analyzed using paired t test and ANOVA in SPSS.
4. Results
The initial concentration of iodine in the samples was less than the upper limit standard (40 ppm), while it was less than the lower limit standard (30 ppm) in salt 3.
The concentration of iodine was totally analyzed for 240 times over a period of three months at ambient light and darkness, humidity and non-humidity, ambient temperature, refrigerator temperature and temperature of 37°C every 15 days.
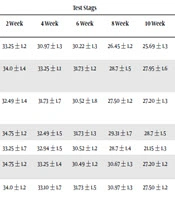
Comparison of initial iodine concentration with its changes in different conditions in samples is presented in Tables 1 - 5.
| Salt Conditions | Test Stags | ||||||
|---|---|---|---|---|---|---|---|
| Initial Concentration of Iodine | 2 Week | 4 Week | 6 Week | 8 Week | 10 Week | Reducing Iodine Concentration | |
| Iodine initial concentration | 36.24 ± 1.1 | 33.25 ± 1.2 | 30.97 ± 1.3 | 30.22 ± 1.3 | 26.45 ± 1.2 | 25.69 ± 1.3 | 10.55 ± 1.2 |
| Refrigerator temperature with humidity | 36.24 ± 1.2 | 34.0 ± 1.4 | 33.25 ± 1.1 | 31.73 ± 1.2 | 28.7 ± 1.5 | 27.95 ± 1.6 | 8.74 ± 1.3 |
| Refrigerator temperature without humidity | 36.24 ± 1.6 | 32.49 ± 1.4 | 31.73 ± 1.7 | 30.52 ± 1.8 | 27.50 ± 1.2 | 27.20 ± 1.3 | 9.04 ± 1.4 |
| 37°C with humidity | 36.24 ± 1.5 | 34.75 ± 1.2 | 32.49 ± 1.5 | 31.73 ± 1.3 | 29.31 ± 1.7 | 28.7 ± 1.5 | 7.54 ± 1.3 |
| 37°C without humidity | 36.24 ± 1.8 | 33.25 ± 1.7 | 32.94 ± 1.5 | 30.52 ± 1.2 | 28.7 ± 1.4 | 21.15 ± 1.3 | 15.09 ± 1.3 |
| Ambient temperature-light-humidity | 36.24 ± 1.1 | 34.75 ± 1.2 | 33.25 ± 1.4 | 30.49 ± 1.2 | 30.67 ± 1.3 | 27.20 ± 1.2 | 9.04 ± 1.4 |
| Ambient temperature-light-without humidity | 36.24 ± 1.8 | 34.0 ± 1.2 | 33.10 ± 1.7 | 31.73 ± 1.5 | 30.97 ± 1.3 | 27.50 ± 1.2 | 8.74 ± 1.4 |
| Ambient temperature-darkness-humidity | 36.24 ± 1.8 | 35.05 ± 1.5 | 34.45 ± 1.2 | 34.0 ± 1.7 | 33.25 ± 1.2 | 30.67 ± 1.3 | 5.57 ± 1.3 |
aValues are expressed as mean ± SD.
| Salt Conditions | Test Stags | ||||||
|---|---|---|---|---|---|---|---|
| Initial Concentration of Iodine | 2 Week | 4 Week | 6 Week | 8 Week | 10 Week | Reducing Iodine Concentration | |
| Iodine initial concentration | 37.48 ± 1.2 | 34.0 ± 1.9 | 33.25 ± 1.5 | 31.73 ± 1.5 | 29.46 ± 1.7 | 28.70 ± 1.3 | 8.78 ± 1.3 |
| Refrigerator temperature with humidity | 37.48 ± 1.8 | 35.05 ± 1.2 | 34.75 ± 1.7 | 33.25 ± 1.7 | 29.77 ± 1.1 | 29.46 ± 1.3 | 8.02 ± 1.4 |
| Refrigerator temperature without humidity | 37.48 ± 1.8 | 34.75 ± 1.7 | 33.25 ± 1.3 | 32.94 ± 1.4 | 30.22 ± 1.4 | 29.01 ± 1.6 | 8.47 ± 1.2 |
| 37°C with humidity | 37.48 ± 1.8 | 35.05 ± 1.7 | 34.0 ± 1.2 | 34.0 ± 1.3 | 30.67 ± 1.6 | ± 1.2 27.95 | 9.98 ± 1.2 |
| 37°C without humidity | 37.48 ± 1.8 | 32.49 ± 1.5 | 31.73 ± 1.7 | 30.67 ± 1.6 | ± 1.6 28.70 | 23.42 ± 1.2 | 14.06 ± 1.3 |
| Ambient temperature-light-humidity | 37.48 ± 1.9 | 34.40 ± 1.7 | 34.00 ± 1.5 | 31.73 ± 1.3 | 30.22 ± 1.4 | 28.70 ± 1.6 | 8.78 ± 1.3 |
| Ambient temperature-light-without humidity | 37.48 ± 1.3 | 34.75 ± 1.4 | 34.30 ± 1.7 | ± 1.1 33.55 | 30.22 ± 1.2 | 29.46 ± 1.7 | 8.02 ± 1.4 |
| Ambient temperature-darkness-humidity | 37.48 ± 1.4 | 36.26 ± 1.6 | 35.96 ± 1.7 | 35.50 ± 1.3 | 33.55 ± 1.4 | 32.94 ± 1.2 | 4.54 ± 1.4 |
aValues are expressed as mean ± SD.
| Salt Conditions | Test Stags | ||||||
|---|---|---|---|---|---|---|---|
| Initial Concentration of Iodine | 2 Week | 4 Week | 6 Week | 8 Week | 10 Week | Reducing Iodine Concentration | |
| Iodine initial concentration | 18.13 ± 1.8 | 15.86 ± 1.7 | 12.39 ± 1.5 | 12.09 ± 1.4 | 12.09 ± 1.3 | 10.58 ± 1.2 | 7.55 ± 1.3 |
| Refrigerator temperature with humidity | 18.13 ± 1.8 | 16.62 ± 1.9 | 13.15 ± 1.7 | 12.40 ± 1.1 | 12.09 ± 1.2 | 11.33 ± 1.3 | 6.80 ± 1.4 |
| Refrigerator temperature without humidity | 18.13 ± 1.8 | 13.75 ± 1.7 | 12.85 ± 1.1 | 11.03 ± 1.2 | 10.58 ± 1.5 | 10.27 ± 1.8 | 7.86 ± 1.4 |
| 37°C with humidity | 18.13 ± 1.8 | 14.35 ± 1.3 | 13.60 ± 1.5 | 12.85 ± 1.8 | 12.85 ± 1.7 | ± 1.6 | 9.59 ± 1.5 |
| 37°C without humidity | 18.13 ± 1.8 | 12.09 ± 1.8 | 12.09 ± 1.7 | 10.88 ± 1.6 | 10.27 ± 1.2 | 6.50 ± 1.3 | 11.63 ± 1.3 |
| Ambient temperature-light-humidity | 18.13 ± 1.8 | 12.85 ± 1.2 | 12.09 ± 1.3 | 11.33 ± 1.7 | 10.58 ± 1.5 | 9.06 ± 1.2 | 9.07 ± 1.3 |
| Ambient temperature-light-without humidity | 18.13 ± 1.8 | 15.11 ± 1.7 | 14.35 ± 1.2 | 13.60 ± 1.4 | 11.33 ± 1.3 | 10.58 ± 1.4 | 7.55 ± 1.4 |
| Ambient temperature-darkness-humidity | 18.13 ± 1.8 | 16.62 ± 1.3 | 15.11 ± 1.4 | 14.05 ± 1.2 | 13.60 ± 1.3 | 12.54 ± 1.2 | 5.59 ± 1.3 |
aValues are expressed as mean ± SD.
| Salt Conditions | Test Stags | ||||||
|---|---|---|---|---|---|---|---|
| Initial Concentration of Iodine | 2 Week | 4 Week | 6 Week | 8 Week | 10 Week | Reducing Iodine Concentration | |
| Iodine initial concentration | 36.26 ± 1.2 | 32.94 ± 1.3 | 30.97 ± 1.2 | 28.70 ± 1.3 | 27.20 ± 1.4 | 26.0 ± 1.3 | 10.26 ± 1.3 |
| Refrigerator temperature with humidity | 36.26 ± 1.7 | 34.0 ± 1.8 | 33.25 ± 1.7 | 31.73 ± 1.3 | 28.70 ± 1.5 | 27.65 ± 1.3 | 8.61 ± 1.3 |
| Refrigerator temperature without humidity | 36.26 ± 1.3 | 33.25 ± 1.2 | 31.73 ± 1.8 | 29.92 ± 1.5 | 27.95 ± 1.7 | 9.06 ± 1.4 | |
| 37℃-with humidity | 36.26 ± 1.5 | 34.45 ± 1.8 | 33.25 ± 1.7 | 31.73 ± 1.3 | 29.46 ± 1.5 | 28.70 ± 1.2 | 7.56 ± 1.3 |
| 37℃ without humidity | 36.26 ± 1.5 | 34.75 ± 1.2 | 34.75 ± 1.5 | 34.0 ± 1.2 | 29.46 ± 1.5 | 22.36 ± 1.7 | 13.90 ± 1.3 |
| Ambient temperature-light-humidity | 36.26 ± 1.5 | 35.50 ± 1.7 | 35.20 ± 1.6 | 34.60 ± 1.5 | 30.22 ± 1.7 | 24.18 ± 1.8 | 12.08 ± 1.4 |
| Ambient temperature-light-without humidity | 36.26 ± 1.8 | 35.05 ± 1.5 | 34.75 ± 1.7 | 34.0 ± 1.3 | 31.73 ± 1.5 | 27.97 ± 1.7 | 8.31 ± 1.3 |
| Ambient temperature-darkness-humidity | 36.26 ± 1.7 | 35.96 ± 1.9 | 35.50 ± 1.4 | 34.45 ± 1.6 | 30.97 ± 1.7 | 30.22 ± 1.3 | 6.04 ± 1.3 |
aValues are expressed as mean ± SD.
| Salt Conditions | Test Stags | ||||||
|---|---|---|---|---|---|---|---|
| Initial Concentration of Iodine | 2 Week | 4 Week | 6 Week | 8 Week | 10 Week | Reducing Iodine Concentration | |
| Iodine initial concentration | 37.02 ± 1.5 | 31.73 ± 1.7 | 30.52 ± 1.4 | 29.46 ± 1.6 | 29.01 ± 1.7 | 25.70 ± 1.5 | 9.52 ± 1.3 |
| Refrigerator temperature with humidity | 37.02 ± 1.7 | 34.75 ± 1.6 | 33.25 ± 1.5 | 32.18 ± 1.3 | 29.46 ± 1.2 | 27.50 ± 1.1 | 9.82 ± 1.3 |
| Refrigerator temperature without humidity | 37.02 ± 1.6 | 33.25 ± 1.8 | 31.73 ± 1.7 | 30.97 ± 1.9 | 27.80 ± 1.5 | 27.20 ± 1.4 | 9.82 ± 1.3 |
| 37°C with humidity | 37.02 ± 1.1 | 35.50 ± 1.3 | 34.75 ± 1.8 | 33.25 ± 1.1 | 30.82 ± 1.2 | 28.70 ± 1.7 | 8.32 ± 1.3 |
| 37°C without humidity | 37.02 ± 1.5 | 33.25 ± 1.7 | 32.94 ± 1.6 | 32.02 ± 1.3 | 29.46 ± 1.7 | 21.90 ± 1.4 | 15.12 ± 1.4 |
| Ambient temperature-light-humidity | 37.02 ± 1.2 | 34.30 ± 1.7 | 34.0 ± 1.4 | 33.25 ± 1.6 | 31.43 ± 1.2 | 27.95 ± 1.1 | 9.07 ± 1.3 |
| Ambient temperature-light-without humidity | 37.02 ± 1.7 | 34.75 ± 1.6 | 34.0 ± 1.7 | 33.25 ± 1.6 | 30.97 ± 1.8 | 26.45 ± 1.2 | 10.57 ± 1.4 |
| Ambient temperature-darkness-humidity | 37.02 ± 1.7 | 35.26 ± 1.3 | 35.35 ± 1.7 | 34.75 ± 1.4 | 33.25 ± 1.5 | 31.73 ± 1.8 | 5.29 ± 1.3 |
aValues are expressed as mean ± SD.
5. Discussion
The results showed that the initial iodine concentration in all studied samples was less than 40 ppm, approved by the Control Committee for preventing IDDs, especially in salt 3, which was less than the minimum concentration of 30 ppm (its initial concentration was 18.13 ± 1.8 ppm).
Moreover, it declined in all conditions over three months. In addition, iodine concentration in all conditions had the highest reduction in salt 5 and the lowest reduction in salt 3.
Besides, there was no significant difference between iodine reduction and salt type (P = 0.52). In the current study, the amount of iodine was less than the minimum concentration in samples 5, 4, 2, and 1 at all stages except in ambient temperature-dark-non-humidity condition. However, in sample 3, initial concentration of iodine was less than the minimum standard, and was reduced in the next stages.
In all samples, the highest reduction of iodine was related to ambient temperature-light-humidity condition (5) with a mean decrease of 13.96 ± 1.3 ppm and the lowest one belonged to ambient temperature-dark-non-humidity condition with a mean decrease of 5.41 ± 1.3 ppm, indicating the effect of light and humidity on removing iodine from salt. In addition, there was a significant difference between the ambient temperature-light-humidity (13.96 ± 1.3 ppm) and ambient temperature-light-non-humidity conditions (9.6 ± 1.3 ppm) (P = 0.001).
Moreover, there was a significant difference between the ambient temperature-dark-humidity (8.64 ± 1.3 ppm) and the ambient temperature-dark-non-humidity conditions (5.41 ± 1.3 ppm) (P = 0.036).
Considering that humidity was the only variable in conditions (1, 2) - (3, 4) - (5, 6) and (7, 8), it can be said that these differences occurred due to humidity. In other words, iodine concentration reduced more significantly in humidity than non-humidity condition.
In addition, iodine reduction was higher in ambient temperature-light-humidity (13.96 ± 1.3 ppm) than that in ambient temperature-darkness-humidity condition (8.64 ± 1.3 ppm) (P = 0), which was statistically significant. The iodine reduction was significantly higher in ambient temperature-light-non-humidity (9.6 ± 1.3 ppm) than that of ambient temperature-dark-non-humidity condition (5.41 ± 1.3 ppm) (P = 0.002).
Given that only the light and dark were variable factors in conditions (5, 7) and (6, 8), it can be said that light was responsible for reducing iodine in samples.
Moreover, iodine reduction was higher in refrigerator temperature-humidity condition (9.69 ± 1.3 ppm) than that in the temperature of 37°C with humidity (8.85 ± 1.3 ppm) (P = 0.988) and was higher in refrigerator-temperature-non-humidity condition (8.34 ± 1.4 ppm) than that in the temperature of 37°C without humidity (7.8 ± 1.3 ppm) (P = 0.999). Considering that the only variable was temperature in conditions (1, 3) and (2, 4), it can be said that iodine reduction was higher in refrigerator temperature than that in 37°C, but this relationship was not statistically significant.
In addition, in all studied salt samples, iodine reduction was the highest in ambient temperature-light-humidity condition (13.96 ± 1.3 ppm) and the lowest in ambient temperature-dark-non-humidity condition (5.41 ± 1.3 ppm) (P = 0).
Therefore, based on all findings, it can be concluded that the best condition for salt storage is ambient temperature, dark and non-humid. The results of the current study are the same as those of a study in 2008 (14).
Besides, the findings of this study are consistent with those of Mahdinia and Nasehinia who suggested salt with iodine concentration less than 40 ppm should not be sold (15).
Dasgupta et al. (11) in the United States demonstrated that storage at high humidity significantly reduced iodine content, which agrees with the results of the present study.
Similar to the current study, Biber et al. (10) illustrated that about 58.5% iodine of salt was lost at room temperature with a relative humidity of 45% - 30% in sealed bags after three years.
The results of a study indicated that iodine stability of salt is higher in autumn and winter due to the lower humidity, which is consistent with those of the present study (16). Another study suggested that high humidity led to loss of iodine in iodized salt (17).
On the other hand, the results of the current study are inconsistent with the study of Hassanzadeh Khayyat and Jalali Moghaddam Shari (18) who reported slight changes in iodine content of salt in different conditions of humid, non-humid, light, dark and at different temperatures over 8 months.
Thus, in order to prevent the reduction of iodine in salt, it should be stored in a dry and dark place at ambient temperature. Special attention should be paid to the standard amounts of iodine in salt. Further studies are recommended with longer study duration in order to obtain better results.