Dear Editor,
Novel coronavirus which is known as sever acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is emerged in December 2019 from Wuhan, Hubei Province, China for the first time. The SARS-Cov 2 (The coronavirus disease 2019) is etiological cause of a viral sever pneumonia (1); however, The WHO is announced this disease as pandemic in 11th March 2020, due to its quickly spread and increasing number of COVID-19 patients throughout the worldwide (2, 3). There is an increasing number of people death related to COVID-19 which can be result from limitation in the efficient therapeutic options against COVID-19 (4). Introduction of efficient and safe therapeutic agents for COVID-19 is considered as one of the major global concern in this regard (5, 6). There is numerous treatment option for COVID-19 which are including teicoplanin, chloroquine/hydroxychloroquine, remdesivir, lopinavir/ritonavir, oseltamivir, umifenovir, ribavirin, favipiravir, IFN‐alpha, mycophenolic acid, corticosteroids, as well as, angiotensin‐converting enzyme 2 gene‐based peptides; although, there is remains a question that what is therapeutic agents has more virological and clinical efficacy without serious side-effects?? (7, 8).
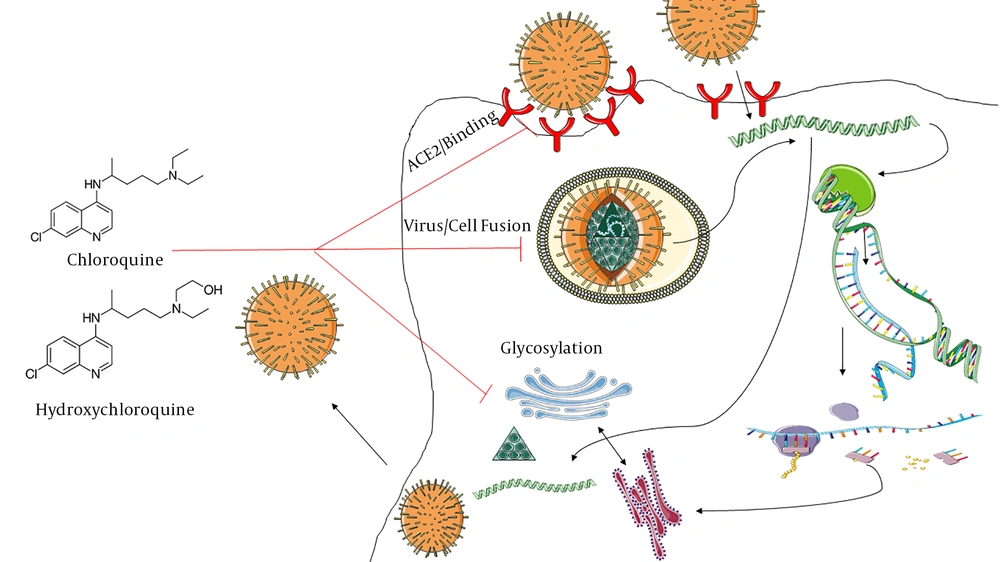
The lopinavir/ritonavir was introduced as the first-line treatment of COVID-19; However, Cao et al. (9) were found that there are no beneficial findings for treatment of adults hospitalized with severe COVID‐19 with lopinavir/ritonavir regimens. In recently days, chloroquine (CQ) has introduced as potential option which can inhibits SARS-CoV 2 in vitro; Wang et al. (10), were showed that CQ has inhibitory effects against SARS‐CoV‐2 infected Vero‐E6 cell lines with EC50 = 1.13 µM concentration. Moreover, there are several documents about effects of CQ on increase of endosomal pH, dysregulation of glycosylation of angiotensin‐converting enzyme 2 (ACE2) receptors, reduction of pro-inflammatory cytokines, as well as, prohibition of cytokine storm initiation via increase of T regulatory subset (11-14). CQ is low-cost antimicrobial agents which is approved for prophylaxis of malaria, amebiasis and treatment of autoimmune diseases e.g. rheumatoid arthritis, systemic lupus erythematous, and, type 2 diabetes (7, 15, 16). Huang et al were conducted a randomized clinical trial in relation to efficacy of Chloroquine in treatment of 10 adult-cases with COVID-19; they are suggested that chloroquine has more beneficial effect compared to lopinavir‐ritonavir combination therapy (17). there are numerous clinical trials which conducted for evaluation of clinical efficacy of chloroquine as therapeutic agent against COVID-19 (7, 15). However, there is major concern about toxicity profile of chloroquine (18). Hydroxychloroquine (HCQ) is less-toxic CQ-derivative option which is emerged as therapeutic agent for SARS-CoV 2 by Yao et al. (19) (EC50 = 0.72µM against SARS‐CoV infected Vero cells); it has been estimated that loading dose of HCQ 400 mg BD is reliable for SARS‐CoV‐2 (Figure 1).
Iran, is developing countries which is located in Middle-East region, according to Iranian Ministry of health reports, there are more than 222,669 COVID-19 patients and 10,508 related-death. According to review of the literatures, Chloroquine is introduced as the first-line treatment of COVID-19 for Iranian patients (20). There is limited documents about efficacy of chloroquine in this country; the aim of present study was evaluating the clinical cure of chloroquine in an Iranian population.
We performed a computer-assisted literature search up to June 2020 to obtain total of available documents about efficacy of chloroquine in Iranian COVID-19 patients using search in several databases including PubMed, Scopus, Google Scholar, Cochrane, Embase, medRxiv, bioRxiv, Science Direct, CNKI, SID, IranMedex, Magiran, and, ISC by keywords according to MeSH keywords which consisting “COVID‐19”, “SARS-CoV 2”, “coronavirus disease 2019”, “chloroquine”, “hydroxychloroquine” and “Plaquenil”. We considered all available documents that published in both of English and Persian languages. The clinical efficacy and adverse event of chloroquine treatment in Iranian COVID-19 patients were assessed by Comprehensive Meta-Analysis (CMA) software version 2.2 (Biostat, Englewood, NJ, USA) using Event rate; heterogeneity were calculated using I2 index and Cochrane Q test (P < 0.05) In addition, the publication bias was also estimated via Beggs and Mazumdar rank and Egger’s regression model.
Overall, 5 articles (total participants = 345) were included in the present analysis (21-25). The majority portion of COVID-19 patients were treated by chloroquine, oseltamivir, and, lopinavir/ritonavir according to Iranian Ministry of health guidelines as well as some minor modifications (20, 24, 26). We estimated that clinical efficacy of chloroquine/hydroxychloroquine supplementation was 72.3% (65.3% - 78.4%); P value: 0.001; Q value: 0.96; I2: 0.00; P value: 0.81; Egger’s P value: 0.165; Begg’s P value: 0.308. whereas, adverse event rate e.g. symptom relapse, readmitted to the hospital, re-infection with SARS-CoV 2 and death was assessed about 32.8% (25.6% - 40.9%); P value: 0.001; Q value: 7.17; I2: 44.23; P value: 0.124; Egger’s P value: 0.45; Begges P value: 0.91. Its seems that chloroquine/hydroxychloroquine combination has beneficial results against SARS-CoV 2 among Iranian-COVID-19 patients; although we showed that adverse events of chloroquine/hydroxychloroquine was about 32.8%. But our analysis has several limitations such as (1) limit sample size; (2) low number studies; (3) lack of findings in relation to types of side-effects or reduction virological load, duration of cough, body temperature; and (4) lack of evidence for evaluation of CQ/HCQ efficacy compared with other therapeutically options (control/conventional/standard group). But efficacy of therapeutic options against COVID-19 should be monitored regularly to better patient management and increase of patient survival and prevention of mortality caused by SARS-CoV 2. It necessary to preform further investigation with more sample size.
Chloroquine has been emerged as treatment option of SARS-CoV 2 in several region of the world (15). The Taiwan CDC was announced that hydroxychloroquine is considered as therapeutically options against COVID-19 except in treatment of patients with allergy to HCQ and pregnant or breastfeeding females (15, 27). Moreover, Singh et al. (16), has been proposed that chloroquine/hydroxychloroquine is approved for COVID-19 in India. Recently, Iranian ministry of health was declaring chloroquine as low-cost antimicrobial drugs as the first-line of treatment of SARS-CoV 2 (26). Sarma et al. (28), were performed a meta-analysis to determine the efficacy of Hydroxychloroquine in virological and clinical cure of patients with COVID-19; they are showed that HCQ has preventive effects on radiological progression of lung disease and efficacy and safety of HCQ + azithromycin against COVID-19; however, Sarma et al. (28), were reported that HCQ have no effect on reduction of virological load. Shamshirian et al. (29), in recent meta-analysis were founded that controversial results, they are declared HCQ treatment with/without azithromycin for COVID-19 patients were not clinical benefits and associate with higher mortality rate (29). In General, there are limit documents in relation to evaluation of chloroquine/hydroxychloroquine efficacy in COVID-19; numerous registered clinical trials have not been completed and making conclusion about recommendation or rejection of chloroquine/hydroxychloroquine against COVID-19 is need to preform further investigations.