1. Background
Gestational diabetes is a type of glucose intolerance, which occurs during pregnancy and often resolves spontaneously after pregnancy (1, 2). It is the most common metabolic disorder during pregnancy (3), with the prevalence rate reported to be 1 - 14% in different countries (4). The lowest prevalence of gestational diabetes has been estimated at less than 1% in Singapore and Tanzania, while the highest rate is reported to be 14% in India (5). According to a study in Iran, the prevalence of gestational diabetes is 1.3 - 8.9% (6). Notably, the difference in the prevalence of gestational diabetes in different countries is due to ethnic/racial differences, study populations, and the method used for screening and diagnosis (7).
Gestational diabetes is a public health concern, currently affecting a large population of pregnant women and causing short-term and long-term maternal and neonatal consequences (8). The short-term complications of gestational diabetes include cesarean section, preeclampsia, congenital anomalies, fetal macrosomia, stunted growth, underweight, stillbirth, intrauterine injuries, and neonatal metabolic disorders. In addition, the late complications in the mother and infant include the increased risk of type II diabetes mellitus, impaired glucose tolerance, and obesity (2, 7, 9-11). Maternal risk factors for gestational diabetes include obesity, family history of diabetes, infertility treatment, current urinary tract infections, fetal macrosomia, sudden unexpected infant death, premature birth, preeclampsia, gestational diabetes, and advanced maternal age (12-14).
Diagnosis of gestational diabetes is important in terms of preventing diseases and perinatal disorders, while it also has a positive effect on the long-term health consequences of the mother and the child (15). The disease is asymptomatic and associated with multiple complications, which are not identified by the patients due to the absence of physical complications and lack of referral. Therefore, it is essential to diagnose gestational diabetes using effective screening methods (4).
Iran is a developing country with a young population and has a larger proportion of women of childbearing age who are exposed to the disease (16-18). The present study aimed to compare the pregnancy outcomes of pregnant women with gestational diabetes and healthy women. Conducting the study on the target population of the selected hospital is an innovation.
2. Objectives
The present study aimed to compare pregnancy outcomes in diabetic and healthy women.
3. Methods
This case-control study was conducted on the pregnant women referring to Moatazedi Hospital in Kermanshah, Iran in 2019. The sample population consisted of 380 pregnant women who were selected via census sampling from the available hospital files, including 190 diabetic and 190 non-diabetic pregnant women. Data collection was carried out based on the family records of the subjects.
Data were extracted from the medical records available in the hospital without any rejections. The control group was matched in terms of age and place of residence. The sample size of each group was determined to be 190 cases (total: 380). Data were collected using a checklist including maternal age, body mass index (BMI), gestational age, education level, employment status, and place of residence. The inclusion criterion was diabetic pregnant women.
(1) Neonatal variables: Height, weight, and five- and 10-minute Apgar scores
(2) Maternal variables: Preeclampsia, mode of delivery, delivery outcome, prenatal care, family history of diabetes, history of smoking, prior pregnancies, and congenital anomalies
All ethical issues such as obtaining a consent form were considered by the researchers, the necessary coordination was made with the hospital officials, and the issue of confidentiality was also observed in the agenda. After completing and encoding the data, data analysis was performed in SPSS version 22. Chi-square was used to compare the frequency distribution of the qualitative variables by a frequency table, and the correlations between the variables in the case and control groups were assessed using Fisher’s exact test. In addition, the Mann-Whitney U test was applied to compare the means of the quantitative variables in the case and control groups. In all the statistical analyses, the significance level was set at 0.05.
4. Results
In total, 190 women with gestational diabetes and 190 healthy pregnant women were enrolled in the study (Table 1).
| Variables | Case | Control | P-Value |
|---|---|---|---|
| Mothers’ age | 27.5 ± 62 | 27.49 ± 4.17 | 0.80 |
| Maternal BMI | 26.05 ± 2.97 | 24.79 ± 2.96 | < 0.01 |
| Gestational age (w) | 38.08 ± 1.72 | 37.09 ± 1.57 | < 0.01 |
a Values are expressed as mean ± SD.
The comparison of the mean maternal age showed a significant difference between the case and control groups (P < 0.01) (Table 2).
| Variables | Case | Control | P-Value |
|---|---|---|---|
| Place of residence | < 0.01 | ||
| City | 114 (60) | 121 (63.7) | |
| Village | 76 (40) | 69 (36.3) | |
| Level of education | < 0.01 | ||
| Diploma and less | 159 (83.7) | 155 (81.6) | |
| Academic education | 31 (16.3) | 35 (18.4) | |
| Occupational status | < 0.01 | ||
| Housewife | 184 (96.8) | 183 (96.3) | |
| Employee | 6 (3.2) | 7 (3.7) |
a Values are expressed as No. (%).
The comparison of the case and control groups indicated significant differences in terms of the place of residence (P < 0.01), education level (P < 0.01), and occupation status (P < 0.01) (Table 3).
| Variables | Mothers (Average Rating) | P-Value | |
|---|---|---|---|
| Diabetes | Healthy | ||
| Gestational age | 222.39 | 158.61 | < 0.01 |
| Maternal BMI | 211.32 | 169.68 | < 0.01 |
| Neonatal Apgar score 5 minutes | 195.15 | 185.85 | 0.23 |
| Neonatal Apgar score 10 minutes | 191.86 | 189.14 | 0.61 |
| Neonatalweight | 256.39 | 124.61 | < 0.01 |
| Neonatal height | 210.89 | 168.11 | < 0.01 |
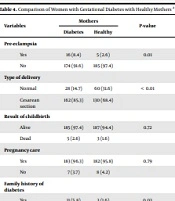
The comparison of the mean gestational age (P < 0.01), maternal BMI (P < 0.01), neonatal weight (P < 0.01), and neonatal height (P < 0.01) also indicated significant differences between the case and control groups (Table 4).
| Variables | Mothers | P-Value | |
|---|---|---|---|
| Diabetes | Healthy | ||
| Pre-eclampsia | |||
| Yes | 16 (8.4) | 5 (2.6) | 0.01 |
| No | 174 (91.6) | 185 (97.4) | |
| Type of delivery | |||
| Normal | 28 (14.7) | 60 (31.6) | < 0.01 |
| Cesarean section | 162 (85.3) | 130 (68.4) | |
| Result of childbirth | |||
| Alive | 185 (97.4) | 187 (94.4) | 0.72 |
| Dead | 5 (2.6) | 3 (1.6) | |
| Pregnancy care | |||
| Yes | 183 (96.3) | 182 (95.8) | 0.79 |
| No | 7 (3.7) | 8 (4.2) | |
| Family history of diabetes | |||
| Yes | 11 (5.8) | 3 (1.6) | 0.03 |
| No | 179 (94.2) | 187 (98.4 | |
| Congenital anomalies | |||
| Yes | 183 (96.3) | 187 (98.4) | 0.34 |
| No | 7 (3.7) | 3 (1.6) | |
| History of pregnancy | |||
| Yes | 62 (32.6) | 74 (38.9) | < 0.01 |
| No | 128 (67.4) | 116 (61.6) | |
| History of smoking | |||
| Yes | 9 (4.7) | 8 (4.2) | < 0.01 |
| N0 | 181 (95.3) | 182 (95.8) | |
a Values are expressed as No. (%).
Our findings also indicated a significant correlation between preeclampsia (P < 0.01), mode of delivery (P < 0.01), family history of diabetes (P = 0.03), prior pregnancies (P < 0.01), and history of smoking (P < 0.01).
5. Discussion
According to the results of the present, the gestational age of the healthy mothers was significantly different from the gestational age of the diabetic mothers whose gestational age was lower than the healthy mothers. This is consistent with the previous findings in this regard (17). Furthermore, the BM of the pregnant women had a significant difference between the case and control groups, which is also consistent with the previous studies in this regard (19-21).
According to the current research, the outcomes of birth weight and neonatal height were significantly different between the mothers with gestational diabetes and the healthy women, and the neonates of the mothers with gestational diabetes were overweight and taller than the infants of the healthy mothers. In addition, the ratio of cesarean sections of mothers in this category confirms this reason. The findings of the current research in this regard are consistent with previous studies (22).
The results of the present study indicated a significant difference between the diabetic and healthy women in terms of preeclampsia, which is consistent with another research in this regard (20). Furthermore, the diabetic and healthy women significantly differed in terms of the mode of delivery as the women with gestational diabetes showed a higher rate of cesarean delivery compared to the healthy women; this is also in line with the previous findings in this regard (22, 23). Since most of the women with gestational diabetes give birth to overweight neonates and due to the anatomical conditions of these women, the rate of cesarean section is often higher among these women than normal subjects.
The results of the present study indicated that the family history of type II diabetes mellitus had a significant difference between the women with gestational diabetes and the healthy women. This finding is consistent with the previous studies in this regard. According to the current research, the family history of diabetes was identified as a risk factor for gestational diabetes, which is in line with other studies. This finding also emphasizes the role of genetics in susceptibility to gestational diabetes. On the other hand, some studies have indicated genetics to be an independent risk factor, especially in individuals aged more than 30 years (24, 25).
The results of the present study showed no significant differences in the five- and 10-minute Apgar scores of infants between the healthy women and the women with gestational diabetes, which is consistent with the results of a similar study (1). Moreover, the two groups in our research were compared in terms of congenital malformations, and no significant difference was observed in this regard. This finding is also in line with some of the previous studies in this respect (23, 26).
The findings of the current research indicated a significant difference between the case and control groups in terms of smoking, which is inconsistent with other studies (27). On the other hand, a significant difference was observed between the two groups in terms of prior pregnancies as the proportion of primiparous healthy women was higher than those with gestational diabetes who experienced their second or multiple pregnancies. This is in line with the findings of similar studies (20).
5.1. Limitations of the Study
The main limitation was that the present study was conducted in only one hospital. Therefore, it is suggested that similar studies target a larger number of hospitals.
5.2. Suggestions
- Taking educational measures to enhance general maternal and neonatal health
- Investment of authorities in maternal and neonatal health
- Interventions to reduce and control gestational diabetes
- Studies on larger sample sizes to investigate gestational diabetes
5.3. Conclusions
According to the results, gestational diabetes was associated with an increased rate of complications, which must be diminished through disease prevention and control to promote maternal and neonatal health.