1. Context
Nonalcoholic fatty liver refers to fat accumulation (over 5%) in the liver cells due to alcohol consumption, hepatitis C, and drugs (1-5). NAFLD is classified into two groups, including nonalcoholic fatty liver and nonalcoholic steatohepatitis. The former case is a benign fat accumulation in hepatic cells with no progress. However, the histopathology of NASH led to the hepatic cells damage and inflammation. The progress to hepatic cirrhosis and even HCC in such cases is probable (6-10). This disease is accompanied by obesity, diabetes, dyslipidemia, and other symptoms of metabolic syndrome.
Nonalcoholic fatty liver should be considered in cases of minimal alcohol consumption (6, 11). The NAFLD includes nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). Furthermore, NAFL is considered one of the most common reasons to develop chronic liver disease, as well as one of the most significant health problems (7, 10-13). Further, NAFLD is related to obesity, diabetes, dysplasia, and hypertension involving liver manifestations in metabolic syndrome. NAFLD histology has been identified as hepatic steatosis and divided into nonalcoholic fatty liver and nonalcoholic steatohepatitis. NAFL is often considered a benign clinical condition without progress, while NASH can lead to cirrhosis and HCC carcinoma. Additionally, NASH is histologically distinguished by liver steatosis, which may lead to liver cell damage and inflammation (10-14).
2. Methods
The evidence reported in the current review was obtained through searching for publications available on MEDLINE, ISI Web of Science, and EMBASE through January 2022 using the following keywords: “Nonalcoholic fatty liver,” “NAFLD,” “NASH,” “Nonalcoholic Steatohepatitis,” “diagnosis,” “treatment,” and “guideline.” Relevant papers with acceptable quality were considered for this study.
In this review, we evaluated the prevalence of fatty liver disease, its clinical features, diagnosis, and treatment. In addition, we focused on evidence related to lifestyle intervention, medical treatment, surgery, and liver transplantation as various choices in the treatment and management of liver disease.
2.1. Prevalence
The prevalence of NAFLD relies on the studies population and its definition (15-18), which is about 20 - 40% in the Western countries and 12 - 30% in Asian countries. Based on the data related to the annual aging survey, 9 - 30% of Japanese adults were diagnosed with ultrasound (US). NASH occurs in 10 - 20% of the cases of NAFLD, with an estimated prevalence of 3 - 5% worldwide. In addition, NAFLD/NASH is mainly identified in middle-aged postmenopausal women and men (19-27).
The severity of risk factors can lead to more prevalence of NAFLD, 10 - 20% of which are related to non-obese subjects, while about 50% have a BMI of more than 25 kg.m-2 (however, less than 30 kg.m-2), 80% of those have BMI more than 30 kg.m-2. Further, NAFLD is common among about 50% of patients with type 2 diabetes (6).
The number of NAFLD is 20 - 30% of the whole population, while 2 - 3% are recognized with NASH. In addition, NAFLD is more prevalent among people with central obesity, type 2 diabetes, insulin resistance, dyslipidemia, and high blood pressure (7, 8, 28, 29).
2.2. Clinical Features
NAFLD is asymptomatic based on the EASL guideline except for cirrhosis in the liver. Hence, NAFLD should be detected during regular checkups or clinical reviews of other diseases. Most patients with NAFLD are obese, and many have type 2 diabetes, dyslipidemia, and/or high blood pressure. Therefore, NAFLD has not considered a liver disease, but a metabolic syndrome (5). The NAFLD is defined according to the three criteria, including nonalcoholic, detection of steatosis either by imaging or histology, and proper exclusion of other liver diseases. Hence, NAFLD patients have a fatty liver with no inflammation or tissue damage. However, NASH is determined based on steatohepatitis on liver biopsy, which is associated with inflammation and tissue damage.
2.3. Diagnosis
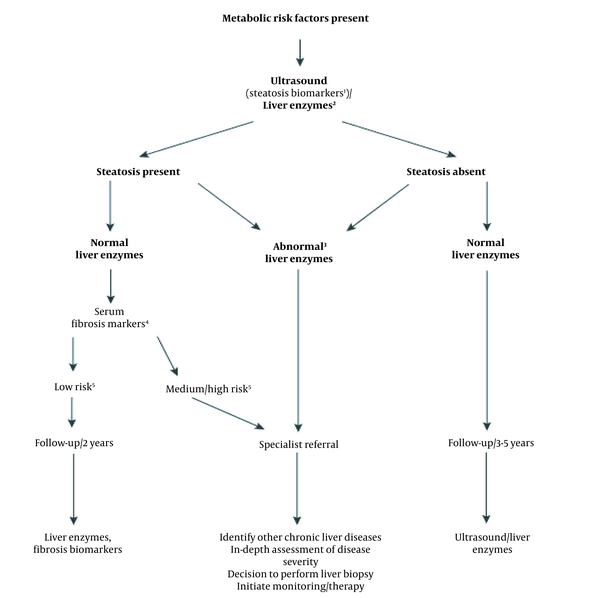
Ultrasonography is the first and most common diagnostic method in NAFLD. The major finding in the ultrasonography analysis is the increased hepatic echogenicity (6). This method does not suffice in ruling out the other causes of steatosis, such as viral and medication-related hepatitis (13). Measurement of the hepatic fibrosis biomarkers, such as FIBS.APRI and elastography are among the other non-invasive methods of NAFLD diagnosis. These methods can help diagnose and evaluate of the fibrosis severity in NAFLD patients and decline the need for biopsy (Figure 1) (5).
Diagnostic flow-chart for evaluating and monitoring the disease severity in the presence of suspected NAFLD and cardio-metabolic factors. (1) Steatosis biomarkers: Fatty Liver Index, Steato Test, NAFLD fat score; (2) Liver tests: ALT, AST or GGT; (3) Any increase in ALT, AST, or GGT; (4) Serum fibrosis markers: NAFLD Fibrosis Score, FIB-4, Commercial tests (e.g., FibroTest, FibroMeter, or ELF scores); (5) Low risk: Showing no/mild fibrosis; medium/high risk indicates significant fibrosis or cirrhosis.
2.4. Biopsy Indication
The indications of biopsy conductance in NASH are: (1) in patients with an increased chance of disease progression, and biopsy should be repeated every five years in these patients; (2) high hepatic enzyme levels, which increases AST/ALT for three months (3, 30); (3) metabolic syndrome, in which patients do not respond to the treatment for 6 months; (4) ruling out the other causes of chronic hepatic diseases (31, 32).
2.5. Treatment
2.5.1. Lifestyle Intervention
About 3 - 5% weight loss can effectively improve steatosis, and 25% calorie reduction can also be included. Physical activities should be 3 - 4 times a week in 30 - 60 min fast walking (14, 32).
2.5.2. Medical Treatment
2.5.2.1. Vitamin E
Vitamin consumption (800 IU/day) is accompanied by reduced hepatic enzymes and histological improvements (33-37).
Some studies reported the non-effectiveness of vitamin E among diabetic patients (32).
WJG and EGO guidelines did not mention vitamin E administration (Table 1).
| Variables | WGO GUIDELINE | WJG | EASL | Brazil |
|---|---|---|---|---|
| Thiazolidine | Not recommend (14) | NASH patients, particularly with fibrosis (≥ stage F2) (13) | It is utilized for the selected NASH patients (5) | It is used for treating steatohepatitis diagnosed through biopsy (31) |
| Metformin | Not recommend (14) | Not recommend (13) | Little evidence is available for a histological effect of metformin in NASH (5) | Not recommend (31) |
| Vitamin E | Not recommend (14) | Not recommend (13) | Vitamin E (800 IU/day) can play a role in improving steatosis, inflammation, ballooning, and induced resolution among 36% NASH patients (5) | Vitamin E (800IU/day) is suggested for the patients by diagnosing histologically; No evidence is available for suggesting vitamin E for diabetic patients (31) |
| Ursodeoxycholic acid (UDCA) | - | - | Indicated some biochemical without any histological improvement (5) | Not recommend (31) |
Medical Treatment Based on Guidelines Updates
2.5.2.2. Metformin
AASLD, WJG, EASL, and WGO did not mention histological improvement and reduced hepatic enzymes by metformin consumption (10, 38) (Tables 1 - 2).
| Variables | AASLD | Japan |
|---|---|---|
| Thiazolidine | It is implemented to cure steatohepatitis among biopsy-proven NASH patients (10) | Patients with insulin resistance (6) |
| Metformin | Not recommend (10) | Not recommend (6) |
| Vitamin E | Vit E: Vitamin consumption (800 IU/day) is accompanied by reduced hepatic enzymes and histological improvements (34-36). Vitamin E is not suggested to cure NASH among diabetic patients (10). | Some studies reported the non-effectiveness of vitamin E among diabetic patients. |
| Ursodeoxycholic acid (UDCA) | Not recommend (10) | Not recommend (6) |
Medical Treatment Based on Guideline Updates
2.5.2.3. Pioglitazone
This drug has been recommended in the studied guidelines for diabetic and prediabetic patients suffering from NFLD, but it was not recommended by WGO guidelines (10, 39) (Table 1).
2.5.2.4. HMG-CoA Reductase Inhibitors (Statins)
This drug class has not been recommended in any of the studied guidelines. Japan guidelines only recommended it in NFLD patients with simultaneous dyslipidemia (6, 40-42).
2.5.2.5. UDCA
It is not recommended in any of the guidelines, the European guideline only mentioned partial hepatic enzyme improvement with no effect on the histological improvements (5, 42-46).
2.5.2.6. Pentoxifylline
It is only mentioned in Japanese guidelines and EASL for a slight improvement in histology in stages above or equal to 2. The minor improvement in liver-related complications are perisinusoidal and periportal lesion (stage 2 fibrosis), bridging fibrosis (stage 3 fibrosis), cirrhosis (stage 4 fibrosis) (5, 6, 47-51).
2.5.2.7. Angiotensin II Receptor Antagonist
Angiotensin II receptor antagonists (ARB) are recommended for NASH patients suffering from hypertension (52-54).
2.5.2.8. Iron Depletion
Iron depletion can improve the NAS score without worsening fibrosis (55). The Pathology Committee of the NASH Clinical Research Network designed and proposed a validated histological feature scoring system to determine all spectrum of damages of NAFLD. The scoring system of NAS includes 14 histological characteristics, four of which are assessed semi-quantitatively: (1) steatosis (0 - 3); (2) lobular inflammation (0 - 2); (3) hepatocellular ballooning (0 - 2); and (4) fibrosis (0 - 4). Another nine features are considered to be present or absent (56).
2.5.3. Surgery
2.5.3.1. Bariatric (Metabolic) Surgery
Bariatric surgery is regarded as an alternative for metabolic complications to reduce weight among patients, who cannot adapt to lifestyle changes and pharmacotherapy, along with consistent results during the long term (41-43, 55). Laparoscopic Roux-gastric bypass surgery is valuable and cost-effective in obese patients (BMI ≥ 30 kg/m2) with mild, moderate, and severe NASH in all three stages of fibrosis (F0 - F3), which is only helpful and cost-effective in the F3 stage in overweight NASH patients (BMI: 25 - 29.9 kg/m2).
2.5.3.2. Liver Transplantation
Liver transplantation is considered a suitable procedure for Nonalcoholic steatohepatitis. Patients experiencing liver failure and/or Hepatocellular carcinoma are regarded as the candidates for liver transplantation (49-51).
3. Conclusions
Hepatic cirrhosis can develop from untreated fatty liver disease, which can even progress toward hepatocellular carcinoma as one of the most common hepatic diseases. Laboratory evaluations and ultrasonography in high-risk patients (diabetes, obesity) are crucial in their symptomless nature. Lifestyle change is the primary treatment for this disease, and medications such as vitamin E and pioglitazone are also helpful.