1. Background
Coronavirus infection began in Wuhan (China) in late 2019, which was renamed COVID-19 by the World Health Organization (1). The mortality rate for this pandemic disease is high, almost entirely due to severe pneumonia (2, 3). The immune system plays an essential role in combating a variety of pathogens. Numerous factors, including nutritional factors, have a critical role in immune system strength. Some micronutrients, such as vitamin D and iron, have vital roles (4) and their deficiency is two of the world’s common health problems (5). In addition to its anti-inflammatory, immune-regulating, and hematopoietic effects, Vitamin D is necessary to maintain calcium and phosphorus levels in the body (6). Several studies have shown the relationship between vitamin D deficiency and viral infection outbreaks in winter (2, 4, 7). The numerous physiological effects of vitamin D, as well as its widespread presence in body tissues, including lymphoid and hematopoietic cells, make it a vital component in the development of red blood cells (8). Researchers have found a significant correlation between anemia and serum vitamin D deficiency. In addition, Vitamin D supplementation effectively improves anemia in clinical trials (4, 9). Iron deficiency, whether combined with anemia or not, is related to a higher incidence of infectious disease (8).
Ferritin is an acute-phase reaction protein, which stores iron in cells and releases it when required (10). Inflammatory, chronic, and infectious conditions can elevate serum ferritin levels (11). Patients with COVID-19 have been reported to have cytokine storm syndrome and increased ferritin levels (12). In addition, the elevation of serum ferritin was suggested as a predictor of COVID-19 mortality (13). According to recent research in a murine sepsis model, ferritin light chain protects against sepsis-induced inflammation and organ injury through an inhibitory effect on NF-*B activation (14). In other words, vitamin D reduces the production of inflammatory cytokines by the innate immune system and protects the respiratory system by stabilizing solid structural connections, eliminating accumulated viruses through cathelicidins and defensins activation (15). As a result, this elimination is associated with a lower risk of cytokines storm, leading to pneumonia.
2. Objectives
Nowadays, concerns about adequate vitamin D and iron intake have grown. As a result, studies are needed to research the possible role that these micronutrients play in the severity and mortality of COVID-19 due to their ability to deal with inflammatory responses.
3. Methods
This descriptive-analytical cross-sectional study was conducted in the early stages of COVID-19. There was unclear information about the duration of this pandemic due to the emergence of the disease, and determining the sample size using statistical formulas was not available. Therefore, demographic characteristics, comorbidities, clinical symptoms, and laboratory findings of 437 patients who were hospitalized in Golestan Hospital of Kermanshah, Iran from April to August 2020 were analyzed. Studying many COVID-19 patients and studying the relationship between vitamin D levels in blood and COVID-19 severity is one of the strengths of the present study. The inclusion criteria were participants who signed informed consent forms and the legal guardians of deceased patients. People who were not satisfied with their participation, had incomplete or lost information were excluded from the study. COVID-19 was confirmed by a medical team based on clinical symptoms, CT scans, and PCR test results. The medical team determined the severity of the disease based on the severity of clinical symptoms, as well as the extent and degree of lung infection. A professional nurse took 2 mL of blood from each patient after data collection, and sent blood samples to a central laboratory. Then, serum levels of 1,25 dihydroxy vitamin D3 [1,25 (OH)2D3] and ferritin were measured by Enzyme-linked immunosorbent assay (ELISA) and Chemiluminescence immunoassay (CLIA) methods after serum isolation.
We used SPSS software Version 16.0 for analyzing the data. The mean and standard deviation (SD) were used to describe continuous data, but categorical variables were expressed using numbers and percentages. We also used Kolmogorov-Smirnov tests, independent t-tests, analysis of variance, and Pearson and Spearman correlation to analyze data. The Kolmogorov-Smirnov test indicated a normal distribution for age and vitamin D levels (P > 0.01). The student’s t-test was used to analyze parametric variables, while the Mann-Whitney U test was utilized to examine non-parametric variables. Kendall correlation was used to analyze the relationship between qualitative variables. A P-value of less than 0.05 was statistically significant in all calculations.
4. Results
The study included 437 COVID-19 patients admitted to Golestan Hospital of Kermanshah, Iran. The mean age of the participants was 60.74 ± 16.70 years, and the majority (59.3%) were male. Table 1 lists the baseline demographic and clinical characteristics. According to the patients’ comorbidities, the most common were diabetes type II (15.1%), hypertension (12%), and cardiovascular disease (7.3%). The most frequent presenting symptom was shortness of breath (58.6%), followed by anosmia (55.1%), ageusia (42.1%), fever (36.2%), and cough (35.5%). Almost all patients had CT positivity (88.1%) and PCR positivity (66.6%). The average serum vitamin D levels were 28.86 ± 15.69 ng/mL. Hypovitaminosis D was found in 53% of patients (Vit D < 30 ng/mL), and 15.3% had severe deficiency (Vit D < 10 ng/mL). Vitamin D serum levels were significantly higher in females than males (P < 0.05). The average serum ferritin levels were 125.32 ± 97.99 μg/L for females and 302 ± 73.6 μg/L for males. Most of Iranian laboratories have reported 300 - 400 μg/L as the normal range for serum ferritin in adult males and 150 - 200 μg/L for adult females (11).
| Parameters | Values |
|---|---|
| Sex | |
| Male | 259 (59.3) |
| Female | 178 (40.7) |
| Age | 60.74 ± 16.70 |
| Patients with comorbidity | |
| None | 274 (62.7) |
| Diabetes type II | 66 (15.1) |
| Hypertension | 53 (12.1) |
| Cardiovascular disease | 32 (7.3) |
| Chronic kidney disease | 12 (2.7) |
| ICU admission | |
| No | 371 (84.9) |
| Yes | 66 (15.1) |
| PCR positivity | |
| No | 143 (32.7) |
| Yes | 291 (66.6) |
| CT positivity | |
| No | 51 (11.7) |
| Yes | 385 (88.1) |
| Severity | |
| Mild | 43 (9.8) |
| Moderate | 270 (61.8) |
| Severe | 124 (28.4) |
| Dead | |
| No | 355 (81.2) |
| Yes | 82 (18.8) |
| Fever | |
| No | 279 (63.8) |
| Yes | 158 (36.2) |
| Cough | |
| No | 282 (64.5) |
| Yes | 155 (35.5) |
| Sore throat | |
| No | 321 (73.5) |
| Yes | 116 (26.5) |
| Rhinorrhoea | |
| No | 312 (71.4) |
| Yes | 124 (28.4) |
| Shortness of breath | |
| No | 179 (41.0) |
| Yes | 256 (58.6) |
| Vomiting | |
| No | 333 (76.2) |
| Yes | 104 (23.8) |
| Ageusia | |
| No | 253 (57.9) |
| Yes | 184 (42.1) |
| Anosmia | |
| No | 196 (44.9) |
| Yes | 241 (55.1) |
| Vitamin D serum level (ng/mL) | 28.86 ± 15.69 |
| Severe deficiency | 67 (15.3) |
| Moderate deficiency | 165 (37.7) |
| Sufficient | 205 (46.8) |
| Ferritin serum level of males (μg/L) | 125.32 ± 97.99 |
| < Normal | 10 (0.03) |
| Normal | 198 (76.44) |
| > Normal | 62 (23.93) |
| Ferritin serum level of females (μg/L) | 302 ±73.6 |
| < Normal | 6 (0.03) |
| Normal | 114 (64.04) |
| > Normal | 47 (26.4) |
Demographic and Characteristics and Presenting Symptoms of Study Population a
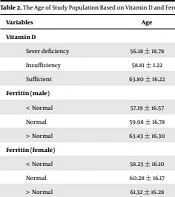
Table 2 represents a significant correlation between age and serum vitamin D levels. Serum vitamin D levels were higher in older patients (P < 0.05), and no significant differences was found between mortality rate, serum vitamin D, and ferritin concentrations. Table 3 represents the clinical symptoms, serum vitamin D, and ferritin levels. A significant correlation was observed between vitamin D levels and severity of the disease, oxygen saturation levels, and rhinorrhea. There was no significant correlation between the incidence rates for fever, sore throat, cough, shortness of breath, vomiting, anosmia, and ageusia. On the other hand, there was a significant difference between ferritin levels and hospitalization duration.
The Age of Study Population Based on Vitamin D and Ferritin Serum Level
| Variables | Vitamin D (ng/mL) | P-Value | Ferritin (μg/L) | P-Value a |
|---|---|---|---|---|
| Sex | 126 | 0.008 a | -0.072 | 0.134 |
| Age | 172 | 0.001 a | -0.075 | 0.119 |
| Patients with comorbidity | -0.074 | 0.125 | 0.03 | 0.538 |
| ICU admission | -0.088 | 0.065 | 0.003 | 0.949 |
| PCR positivity | 0.073 | 0.128 | -0.043 | 0.374 |
| CT positivity | -0.038 | 0.434 | -0.04 | 0.408 |
| Severity | -0.253 | 0.001 a | 0.025 | 0.608 |
| Dead | 0.022 | 0.653 | -0.084 | 0.078 |
| Oxygen saturation | 125 | 0.009 a | -0.068 | 0.154 |
| Fever | -0.065 | 0.176 | 0.053 | 0.272 |
| Cough | -0.088 | 0.065 | 0.072 | 0.133 |
| Sore throat | -0.057 | 0.233 | 0.037 | 0.441 |
| Rhinorrhoea | 0.06 | 0.211 | -0.039 | 0.413 |
| Shortness of breath | 0.042 | 0.378 | -0.039 | 0.547 |
| Vomiting | -0.062 | 0.195 | 0.063 | 0.189 |
| Ageusia | -0.017 | 0.729 | -0.015 | 0.757 |
| Anosmia | -0.062 | 0.197 | -0.015 | 0.523 |
| Hospital Stay | 0.021 | 0.667 | 108 | 0.023 a |
Patient’s Characteristics Based on Vitamin D and Ferritin Serum Levels
5. Discussion
This descriptive-analytical cross-sectional study examined the relationship between serum ferritin, vitamin D levels, and the severity of clinical symptoms in patients with COVID-19. Our study group was divided into three categories based on serum vitamin D and ferritin levels: Vitamin D deficiency was found in 53% of patients (Vit D < 30 ng/mL), of whom 15.3% had severe deficiency (Vit D < 10 ng/mL), and 37.7% had insufficiency. Furthermore, 71.4% were in the normal range of serum ferritin level, and 3.66% and 24.94% were less and more than normal, respectively. According to the European Calcified Tissue Society’s position statement, severe vitamin D deficiency is found in > 10% of Europeans (16). A significant correlation was observed between serum vitamin D levels, lower oxygen saturation rate, and severe COVID-19 diseases, but there was no statistical difference in mortality rate and ICU admission. In Jain‘s study, vitamin D deficiency (Vit D < 20 ng/mL) was far more prevalent, which resulted in ICU admission and thereby increased chances of mortality (8). This rate could be related to the small size of the sample, and thus, larger sample size is required. In this study, there was a significant difference between the serum ferritin levels and hospitalization duration. These results are consistent with a study on 20 patients with COVID-19, which focused on ferritin, a serum inflammatory marker, and showed that patients with severe COVID-19 had higher serum ferritin levels (17).
In spite of the lack of knowledge about the molecular and cellular mechanisms that influence serum ferritin levels, it could be related to the positive acute phase of this protein. In older patients, serum vitamin D levels were significantly higher than those in younger patients. By contrast, Ilie et al. measured vitamin D levels in European countries, which were relatively low in the elderly population, and found a reversed significant correlation between vitamin D levels, COVID-19 cases, and mortality in those populations (18).
We suppose that these results are because of the small number of old patients with severe COVID-19. In this study, the most common presenting symptoms were shortness of breath (58.6%), anosmia (55.1%), and ageusia (42.1%). Many studies have reported that cough and dyspnea are the most symptoms of COVID-19 (19-21). All these factors point to severe damage to lungs tissue in COVID-19 patients.
The first limitation of the present study was related to the observational nature of study in which a cause-and-effect relationship could not be found, and the follow-up data were long-term. The second limitation was the lack of interventional studies with appropriate samples size to detect the role of vitamin D and ferritin in COVID-19 patients. In conclusion, our findings indicated that COVID-19 patients, who were treated in the hospital, had a high prevalence of hypovitaminosis D. The severity of the disease was increased in patients with vitamin D deficiency. As serum ferritin levels were elevated, the hospitalization duration was increased. Further studies should be done to evaluate the roles of vitamin D and ferritin in COVID-19 patients.