Dear Editor,
COVID-19 is caused by the SARS-COV-2 virus. Causing acute respiratory syndrome, this virus has infected tens of millions of people and led to a pandemic. Following that, people were quarantined in their homes. Responses and reactions to COVID-19 appear to be widely different, ranging from asymptomatic or mild to severe conditions and death, among different people and regions (1). Numerous clinical trials worldwide have explored the efficacy of existing medicines against COVID-19, including various antiviral and immunomodulatory drugs (2). In its acute phases, when acute respiratory problems are present, N-Acetyl Cysteine and Famotidine are recommended for faster recovery of patients (3, 4). A wide variety of vaccines have been developed to fight the coronavirus. which include different platforms such as RNA, live-attenuated virus, protein subunit, and viral vector (5).
The present study evaluated and compared complications of COVID-19 vaccines and their severity among subjects taking the first dose of Sinopharm, Bharat, AstraZeneca, and Sputnik V vaccines.
This cross-sectional study was conducted in the Clinical Research Development Center of Shahid Mohammadi Hospital, in Bandar Abbas on April 2021, in Bandar Abbas, Iran, Iran with 270 people after injecting the first dose of Gam-COVID-vac, Oxford–AstraZeneca, and Bharat Biotech (Covaxin), and (Sinopharm) BBIBP-CorV. Since this study was done for the first groups of vaccinations, we used the census method for the study. An online questionnaire was designed to assess demographic information in addition to local and systemic complications of vaccination. Subjects were assessed in terms of local complications as well as systemic complications three days after vaccination.
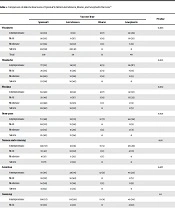
This study was approved by the Research Ethics Committee of Hormozgan University of Medical Sciences. Among 270 vaccinated individuals, fever (62%), anorexia (34%), nausea and vomiting (29%), myalgia (68%), and weakness (82%) were the most prevalent complications in the AstraZeneca group. Furthermore, pain at the vaccination site was also most prevalent in the AstraZeneca group (49/58 (84%)).
Xia et al. and Doroftei et al. reported mild to moderate pain and fever at the injection site as the most common complications of the Sinopharm vaccine, which is consistent with our findings (6, 7). Zhu reported myalgia in 18%, fever in 16%, and nausea and vomiting in 6% of individuals receiving the Sinopharm vaccine. In this study, 20% of people experienced myalgia, 10% experienced fever, and 8% experienced nausea and vomiting after the first dose of the Sinopharm vaccine. In addition, Zhu et al. reported diarrhea in 8% of individuals, which was not reported in the present study (8) (Table 1).
| Vaccine Type | P-Value | ||||
|---|---|---|---|---|---|
| Sputnik V | AstraZeneca | Bharat | Sinopharm | ||
| Weakness | 0.001 | ||||
| Asymptomatic | 63 (53) | 11 (9) | 9 (7) | 34 (29) | |
| Mild | 26 (55) | 8 (17) | 3 (6) | 10 (21) | |
| Moderate | 32 (56) | 19 (32) | 1 (3) | 5 (8) | |
| Severe | 29 (59) | 20 (41) | 0 | 0 | |
| Total | 150 | 58 | 13 | 49 | |
| Headache | 0.031 | ||||
| Asymptomatic | 77 (53) | 19 (13) | 8 (5) | 39 (27) | |
| Mild | 25 (55) | 11 (24) | 2 (4) | 7 (15) | |
| Moderate | 26 (60) | 12 (26) | 3 (6) | 3 (6) | |
| Severe | 22 (58) | 16 (42) | 0 | 0 | |
| Myalgia | 0.012 | ||||
| Asymptomatic | 56 (48) | 18 (14) | 9 (7) | 32 (31) | |
| Mild | 21 (46) | 8 (17) | 3 (6) | 13 (22) | |
| Moderate | 43 (69) | 16 (25) | 1 (1) | 2 (3) | |
| Severe | 30 (62) | 16 (33) | 0 | 2 (5) | |
| Bone pain | 0.021 | ||||
| Asymptomatic | 73 (48) | 23 (15) | 12 (7) | 44 (29) | |
| Mild | 20 (55) | 13 (36) | 0 | 3 (8) | |
| Moderate | 32 (72) | 9 (20) | 1 (2) | 2 (4) | |
| Severe | 25 (65) | 13 (34) | 0 | 0 | |
| Nausea and vomiting | 0.01 | ||||
| Asymptomatic | 124 (57) | 41 (18) | 11 (5) | 45 (20) | |
| Mild | 11 (42) | 10 (32) | 1 (3) | 4 (15) | |
| Moderate | 8 (57) | 5 (35) | 1 (7) | 0 | |
| Severe | 7 (77) | 2 (33) | 0 | 0 | |
| Anorexia | 0.017 | ||||
| Asymptomatic | 111 (57) | 28 (14) | 12 (6) | 45 (23) | |
| Mild | 19 (52) | 16 (43) | 0 | 2 (5) | |
| Moderate | 14 (50) | 11 (39) | 1 (3) | 2 (8) | |
| Severe | 6 (66) | 3 (34) | 0 | 0 | |
| Sneezing | 0.1 | ||||
| Asymptomatic | 139 (57) | 50 (20) | 13 (4) | 45 (19) | |
| Mild | 10 (55) | 4 (22) | 0 | 4 (22) | |
| Moderate | 0 | 4 | 0 | 0 | |
| Severe | 1 | 0 | 0 | 0 | |
| Cough | 0.001 | ||||
| Asymptomatic | 139 (56) | 47 (19) | 12 (4) | 47 (19) | |
| Mild | 8 (47) | 7 (43) | 1 (5) | 1 (5) | |
| Moderate | 3 (37) | 4 (50) | 0 | 1 (5) | |
| Severe | 0 | 0 | 0 | 0 | |
| Sore throat | 0.021 | ||||
| Asymptomatic | 124 (53) | 46 (20) | 12 (5) | 47 (20) | |
| Mild | 12 (54) | 7 (32) | 1 (4) | 2 (9) | |
| Moderate | 11 (78) | 3 (22) | 0 | 0 | |
| Severe | 3 (79) | 1 (25) | 0 | 0 | |
| Fever | 0.017 | ||||
| Asymptomatic | 89 (55) | 18 (9) | 11 (6) | 44 (27) | |
| Mild | 36 (54) | 23 (34) | 2 (3) | 5 (7) | |
| Moderate | 13 (50) | 13 (50) | 0 | 0 | |
| Severe | 12 (75) | 4 (25) | 0 | 0 | |
| Chills | 0.013 | ||||
| Asymptomatic | 92 (57) | 17 (10) | 10 (7) | 45 (26) | |
| Mild | 16 (48) | 12 (35) | 2 (5) | 4 (12) | |
| Moderate | 19 (59) | 12 (37) | 1 (2) | 0 | |
| Severe | 23 (57) | 17 (43) | 0 | 0 | |
| Urticaria | 0.032 | ||||
| Asymptomatic | 143 (55) | 56 (21) | 13 (5) | 48 (18) | |
| Mild | 5 (84) | 0 | 0 | 1 (16) | |
| Moderate | 1 (100) | 0 | 0 | 0 | |
| Severe | 1 (33) | 2 (67) | 0 | 0 | |
| Pain at injection site | 0.002 | ||||
| Asymptomatic | 43 (53) | 9 (12) | 5 (6) | 24 (29) | |
| Mild | 47 (54) | 16 (18) | 5 (5) | 18 (20) | |
| Moderate | 38 (57) | 18 (27) | 2 (3) | 8 (12) | |
| Severe | 23 (60) | 14 (36) | 1 (4) | 0 | |
| Swelling | 0.021 | ||||
| Asymptomatic | 124 (53) | 48 (20) | 13 (5) | 45 (18) | |
| Mild | 13 (56) | 6 (26) | 0 | 4 (18) | |
| Moderate | 10 (90) | 1 (10) | 0 | 0 | |
| Severe | 3 (50) | 3 (50) | 0 | 0 | |
| Discoloration | 0.021 | ||||
| Asymptomatic | 135 (55) | 49 (20) | 13 (5) | 44 (19) | |
| Mild | 14 (73) | 3 (15) | 0 | 2 (10) | |
| Moderate | 1 (33) | 2 (67) | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | |
a Values are expressed as No. (%).
In Doroftei et al.’s study, 3% reported headache, 3% fatigue, 5% pain at injection site, 2% fever, and nausea and vomiting 2% in individuals receiving the first dose of Bharat vaccine while these figures were respectively 38%, 44%, 61%, 15%, and 15% in the present study. Given that Doroftei et al.’s study population was 755 individuals, the difference in the prevalence of complications can be due to our small sample size (7).
Our study showed that among the under-examination vaccines, the most common local and general complications pertained to AstraZeneca, Sputnik V, Sinopharm, and Bharat vaccines, respectively among the first-dose vaccine recipients. Further studies and comparisons for different vaccines are recommended with larger sample sizes in different communities after receiving the full dose of vaccines.