1. Background
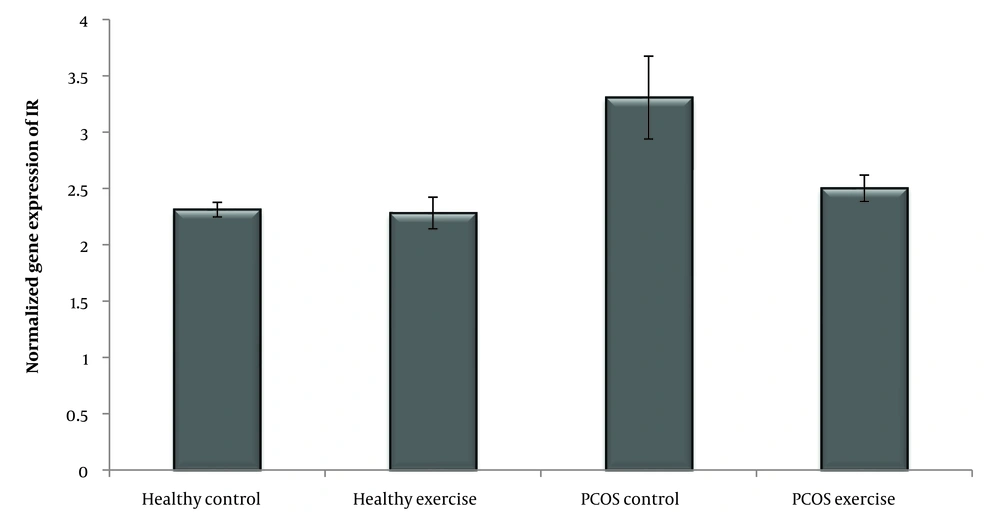
Polycystic ovary syndrome (PCOS) is a highly prevalent condition that affects 5–21% of women globally between the ages of 18 and 45 who are fertile (1). Insulin resistance (IR), central obesity, and dyslipidemia are common conditions associated with PCOS (2). According to reports, women with PCOS have an irregular glucose metabolism brought on by IR or hyperinsulinemia, which increases their risk of developing type 2 diabetes (T2D) by five to seven times compared to normal patients (3). Insulin resistance is identified as an impaired biological response to insulin stimulation of target tissues, primarily the liver, muscle, and adipose tissue (4). The IR happens in the majority (65 - 90%) of obese patients and 25 - 45% in non-obese patients (5). IR has long-term effects on the metabolism of patients with PCOS (3). A diminished response to insulin stimulation characterizes it and inhibits hepatic glucose output (6). IR is an impairment of insulin to mediate metabolism in skeletal muscle, adipocytes, and liver (7).
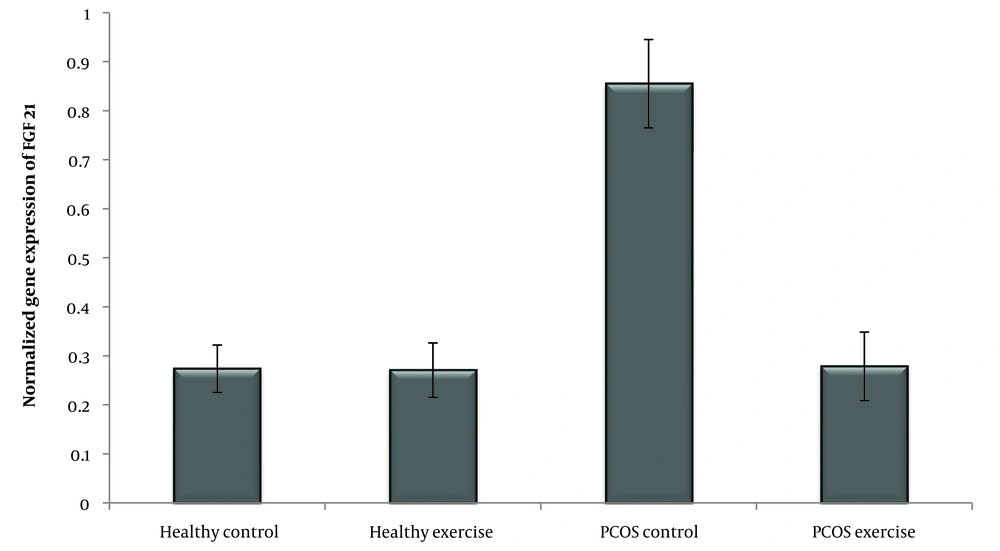
Fibroblast growth factor 21 (FGF-21) is the 21st member of the FGF protein family and plays many biological roles, expressed in liver, muscle, and adipose tissue (8). Studies have shown that FGF-21 levels are increased in T2D, PCOS, metabolic syndrome, obesity, and cardiovascular disorders (9). FGF-21 levels are involved in inflammation and oxidative stress as well as post-exercise changes (10) and increase insulin content in pancreatic β-cells (11, 12). Studies show that FGF-21 is strongly associated with exercise intervention and improves metabolic status (8). However, high resting FGF-21 concentrations indicate resistance to FGF-21 (13). According to the results, long-term exercise reduces circulating FGF-21 levels in obese people (8). Therefore, this information suggests that FGF-21 resistance due to hepatic fat accumulation is an important risk factor for metabolic diseases (13).
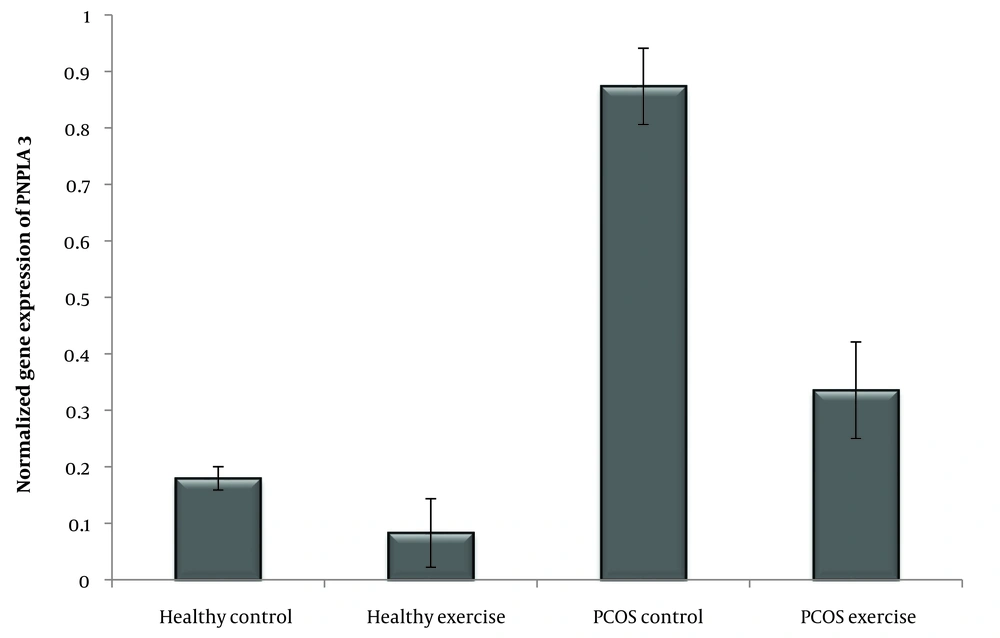
The PNPLA-3 (patatin-like phospholipase domain-contained-3) gene, also known as adipanotrin, is a major determinant of interindividual and racial differences in liver fat content. The results showed that the PNPLA-3 gene is closely related to fat accumulation in hepatocytes (14), plays a major role in the occurrence of none alcoholic fatty liver disease (NAFLD) (15), and is significantly associated with the development of the disease (14). The PNPLA-3 gene encodes a 481 amino acid protein (16) and is highly expressed in liver and adipose tissues (17). The PNPLA3 protein has lipase activity and can hydrolyze triglycerides (18). Hyperandrogenism plays an essential role in fatty liver in women with PCOS (2). This protein is expressed in the endoplasmic reticulum and lipid membrane of liver cells and adipose tissue (14), which may be reversible with weight loss, exercise, and proper diets (6).
2. Objectives
This study aimed to investigate the effect of four weeks of swimming training on insulin resistance and hepatokines in rats with PCOS.
3. Methods
In this experimental study, 24 adult Wistar rats (weighing approximately 180 ± 20 grams) were maintained at 23 ± 2 degrees and a humidity of 40 to 60% with a circadian rhythm of 12 to 12 hours and the same conditions. Additionally, a hormonal stimulation with estradiol valerate (4mg in 0.2 mL sesame oil) was injected intramuscularly into the animals to induce PCOS. Vaginal smear testing is performed daily for sixty days until changes in the estrous cycle and its abnormalities reach the Persistent Vaginal Cornification (PVC) Pap smear stage. After confirmation of polycystic disease, PCOS and healthy mice were randomly divided into four experimental groups (six mice per group). Group 1: Healthy controls. Group 2: PCOS control group. Group 3: Healthy exercise group and group 4: PCOS exercise group. Swimming training is conducted for four weeks, five sessions per week, after two weeks of adaptation to the new environment. According to the procedure, 60 minutes was assigned until the end of the program, and the water flow rate was increased from seven to 15L/M (19) (Table 1). Specialized swimming pool for mice, fiberglass water tank with dimensions: 110 cm length, 35 cm height, 50 cm width, and a capacity of 200 liters with the body material is polyethylene. All rats were anesthetized and operated on by sequential intraperitoneal injection of Ketamine (90 mg/kg) and Xylazine (10 mg/kg) 48 hours after the last training session and after 12 to 14 hours of fasting. Blood samples were measured using enzymatic calorimetry using glucose oxidase technology using a glucose kit (Yakhte Pajohan Sarai, Iran). Fasting serum insulin concentrations were measured using the Elisa Rat Insulin kit manufactured by Elabscience, USA, using the sandwich ELISA method. Regarding sensitivity, the minimum measurement value is 0.19mg/mL, and its detection range is 20 - 30 mg/mL. In addition, the HOMA_IR method was used to calculate the insulin resistance index:
HOMA IR = (Fasting insulin (μU/mL) × Fasting blood sugar (mmol/L)) 22.5.
| Days | Duration (Min) | ||
|---|---|---|---|
| Water Speed 7L/M | Water Speed 15L/M | ||
| First Week | Second Week | Third_4th Weeks | |
| First | 10 | 35 | 60 |
| Second | 15 | 40 | 60 |
| Third | 20 | 45 | 60 |
| Fourth | 25 | 50 | 60 |
| Fifth | 30 | 55 | 60 |
Four Weeks of Swimming Training Program
Liver tissue was cut off and stored in a laboratory refrigerator until RNA extraction, and the Roje RT-ROSET kit was used to generate cDNA. Real-time PCR was performed using the ∆∆Ct calculation method (Table 2). The ethical code of this study is IR.IAU.SRB.REC.1400.187.
| Genes | Accession Number | Product Size (bp) | Reverse | Forward |
|---|---|---|---|---|
| PNPLA-3 | NM_001282324.1 | 159 | GCATCCACCACTTCGTCTTTG | CAACATTAACAAGTGCGTCAGAG |
| FGF-21 | NM_130752.1 | 128 | GGTGTCCTGGTCGTCATCTG | GTCTCCTGCTGCCTGTCTTC |
Genes Primer Forwarding
Statistical analysis was performed using SPSS software version 25.0. The normality of the quantitative data was confirmed by the Shapiro-Wilk test. One-way analysis of variance (ANOVA) was used to determine gene expression, and all variables with P < 0.5 were achieved.
4. Results
According to the Shapiro-Wilk test results and the observed significance level, the data distribution of IR, FGF-21, and PNPLA-3 genes in the groups is normal. In addition, the variance homogeneity of the study was estimated by Levine's test. The results of the one-way ANOVA test showed that the level of IR (P = 0.001) and the expression of the FGF-21 (P = 0.001), PNPLA3 (P = 0.001) genes was significant (Table 3) (Figures 1 , 2 and 3).
| Variables | Sum of Squares | DF | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| PNPLA3 | |||||
| Between groups | 2.241 | 3 | 0.747 | 187.254 | 0.001 |
| Within groups | 0.080 | 20 | 0.004 | ||
| Total | 2.320 | 23 | |||
| FGF_21 | |||||
| Between groups | 1.518 | 3 | 0.506 | 109.864 | 0.001 |
| Within groups | 0.092 | 20 | 0.005 | ||
| Total | 1.610 | 23 | |||
| IR | |||||
| Between groups | 4.161 | 3 | 1.387 | 32.056 | 0.001 |
| Within groups | 0.865 | 20 | 0.043 | ||
| Total | 5.026 | 23 |
One-way ANOVA Results
5. Discussion
According to the induction of PCOS, the amount of IR, FGF-21, and PNPLA-3 increased. In this case, several investigations have found that PCOS women exhibited higher levels of fasting insulin, HOMA-IR (20), FGF21 (7), and PNPLA3, which were directly related to liver disease (14). The result shows four weeks of swimming training dramatically lowered the levels of IR and expression of FGF-21 and PNPLA-3 genes. In this sense, Jurczewska et al. showed that physical activity controls IR in women with PCOS (20). Additionally, Jungari et al. demonstrated that 85 - 90% of PCOS-affected women benefit from the combination therapy of physical activity, calorie restriction, and a high-protein diet (21). According to Stine et al., patients with NASH exhibited a significant reduction in serum FGF21 following aerobic exercise training (22) and improved the metabolism in skeletal muscle and liver (7). Studies have shown that serum FGF-21 concentrations in overweight middle-aged women were significantly reduced after a 3-month combined exercise program (resistance and endurance training) (13). Additionally, Matsui et al. found that after aerobic exercise (12 weeks/three times per week), plasma glucose and serum FGF-21 concentrations decreased in overweight/obese men (23). In this case, studies have indicated that exercise is associated with weight loss, BMI, IR, and inflammatory factors, which play a crucial role in reducing inflammation and increasing insulin sensitivity in PCOS patients (23-25) and FGF-21 as a hepatokine during aerobic exercise (26) regulates carbohydrate, lipid, and energy metabolism (22) that seems similar findings appear in our study. Researchers showed that five weeks of resistance training increased VO2max and decreased liver fat content, reducing FGF-21 levels (13). Further, Matsui et al. show that exercise training reduces plasma glucose and serum FGF21 concentrations (23). Nevertheless, the source of FGF21 is essential, and in this study, we confirmed that the expression of FGF21 was reduced in the liver. Additionally, Shabkhiz et al. demonstrated 12 weeks of resistance training-induced FGF-21 in older men with and without T2DM (27). However, previous studies have shown that the type of exercise, intensity (28), and duration (29) are effective in PCOS. Ma et al. demonstrate that, in mice suffering from myocardial infarction, different types of exercise training enhance the production of FGF-21 (30). Also, Jürimäe et al. show single endurance rowing training significantly increases FGF-21 levels in national-level female rowers (31). Meanwhile, the results of Motahari Rad et al. showed that 12 weeks of concurrent training did not influence FGF-21 of T2DM patients (32). Concurrent training probably sensitizes FGF-21 actions without changing the concentrations of FGF-21 in T2DM patients and could also relate to the training type. In addition, regular endurance exercise also reduces liver fat content and improves FGF-21 resistance (13), but the changes are different for different people during the same exercise (8). Moreover, Long-term (12 weeks) (33) and short-term (3 days) (34) physical interventions increased the expression of KLB, FGFR1, and FGFR2 in obese mice (33). Studies have revealed that exercise lowers blood sugar, which affects the circulation of FGF21 (23) and improves FGF-21 resistance (34). Furthermore, PNPLA-3 plays a vital role in the occurrence of NAFLD (17). In this case, Cinque et al. showed the PNPLA3 GG genotype abolished the effect of lifestyle and appeared as an independent risk factor for weight gain and NAFLD (35). In addition, Wang et al. concluded that physical activity and inactivity may modify the effects of PNPLA-3 type on NAFLD in children (36). Swimming can effectively improve insulin sensitivity and even prevent IR by influencing the expression of proteins involved in lipid metabolism and insulin signaling (37). During aerobic exercise, biochemical adaptations induce a cascade of physiological stimuli that increase oxygen uptake, free fatty acid oxidation, and circulating glucose as an energy source (38). Exercise provides positive clinical outcomes for women with PCOS. However, more knowledge about the molecular mechanisms facilitates this improvement (39). Studies have shown the importance of lifestyle in women with PCOS (40), and lifestyle factors such as diet and exercise can alter DNA methylation. Exercise is an effective non-pharmacological strategy to treat PCOS (38) and may reduce clinical symptoms in the long term (41).
5.1. Limitations
Although a decrease in FGF-21 and PNPLA-3 was observed after swimming exercise, morphological examination of the liver provided a more precise connection of the molecular cell layers. Therefore, hepatic NAFLD should be investigated in future research.
5.2. Conclusions
Submaximal endurance swimming training can decrease IR and the expression of FGF-21 and PNPLA-3 genes. Swimming training seems to positively affect PCOS patients by improving the metabolic pathway.