1. Background
There are more than 30 million deaths worldwide due to cardiovascular diseases, and the number is growing rapidly. As a result, cardiovascular diseases will account for 32.5% of all deaths by 2030 (1, 2). The evidence shows that the prevalence of coronary artery diseases and the resulting deaths are increasing in Iran. Some diseases, such as cardiovascular diseases and atherosclerosis, have increased due to mechanical life and unhealthy foods (3). Atherogenesis begins with damage to the endothelium layer, progresses to narrowing and blockage of the arteries, and results in decreased heart muscle blood flow (4). Various treatments, including diet, lifestyle modification, cardiac rehabilitation, angioplasty, and coronary artery bypass grafting (CABG), are provided in coronary artery patients, and many of them undergo CABG. In many cases, this surgical procedure is the only way to treat and increase life expectancy in these patients (5). Hemorheological disorders, decreased hemoglobin, and hematocrit are some complications observed after CABG (2). Hemorheological conditions are considered the primary cause of the development and spread of cardiovascular diseases. As well as being a cause of ischemic heart disease and atherosclerosis, they also contribute to their development. Blood circulation and cardiovascular system disorders are often caused by hemorheological problems and disturbances in the blood's fibrinogen, hematocrit, and ESR levels (4). Cardiac rehabilitation is one of the essential measures to be more effective after CABG and reduce hemorheological complications (6). Razzaghi et al. showed that aerobic, resistance, and combined exercises improved (decreased) the concentration of fibrinogen and hematocrit, but ESR increased. The decrease in the combined group was significant compared to the aerobic group. In Pabsiak and Sandor, the reduction of hematocrit and, thus, the reduction of the risk of cardiovascular diseases were mentioned (4, 7, 8).
Ivanov concluded that a high level of fibrinogen can be considered one of the main risk factors for developing cardiovascular diseases. Plasma fibrinogen levels were significantly increased in inactive subjects compared to physically active subjects. Regular physical activity, especially in older adults, can be a natural endogenous regulation of fibrinogen levels and/or a natural supplement supporting continuous drug treatment (9). Nazar Ali et al. found that a double competition period (three stages) significantly increased whole blood viscosity. The number of red blood cells, hematocrit, and hemoglobin increased significantly, and plasma volume and ESR significantly decreased (10). Razzaghi et al. investigated the effectiveness of eight weeks of aerobic exercise and combined cardiac rehabilitation on hemorheological variables of middle-aged men. The results in both aerobic and combined training groups showed that improvement was significant compared to the control group in all hemorheological variables, including fibrinogen concentration (P = 0.001), hematocrit concentration (P = 0.002), and ESR (P = 0.001). In addition, the mentioned hemorheological variables in the combined group significantly decreased compared to the aerobic group (4). Mehrafrazi et al. investigated the effect of eight weeks of aerobic training on hematological variables in middle-aged men. The results showed that the impact of aerobic exercise on hematological variables was insignificant. The current level of aerobic exercise in heart patients alone cannot improve the hematological factors of their blood (11). Fathi et al. investigated the response of hemorheological factors to an about of intense exercise at different times of the day; the statistical analysis of the data showed a significant effect of time of day on blood viscosity responses, Plasma viscosity, hematocrit, fibrinogen, ESR, had no significant impact (12).
The present study was conducted due to the prevalence and complications of cardiovascular diseases and the role of exercise in increasing physiological adaptations, the different and contradictory results of the effects of aerobic and combined exercises, and the limited number of studies comparing the effects of combined and aerobic exercises on hemorheological indicators.
2. Objectives
This study aimed to compare the effect of eight weeks of aerobic and combined training on hemoglobin, hematocrit, fibrinogen, and ESR in middle-aged men undergoing CABG.
3. Methods
This clinical trial was conducted in 2023 on 30 middle-aged male CABG patients attending the cardiac rehabilitation unit in Imam Ali Hospital, Kermanshah City, Iran, who had undergone CABG in the past eight weeks. The sample size of this study was calculated based on a similar study, which evaluated hematological indicators in CABG patients using G power software with an effect size of 0.05 and a test power of 80%. The subjects were randomly selected and divided into three groups of 10 (Lottery method: Assigning a number to each person). The first group included the patients with a passive state (control), and the second and third groups included those who performed aerobic and Combined exercises (intervention). Written informed consent was obtained from the participants after admission and counseling, and they received a complete examination by a cardiologist.
The inclusion criteria were: Middle-aged men undergoing CABG, ejection fraction above 40%, no orthopedic complications or other disease preventing participation in the research, stable cardiac state, lack of pain and high-risk arrhythmia, and consumption of similar medications. The exclusion criteria included the emergence of symptoms or new diseases, voluntary withdrawal from the study, and absence from the exercise sessions for more than two sessions.
Three days before the practice sessions, the subjects completed the health status and research consent forms, and then height, weight, and vital signs were measured. The subjects were asked to use a standard diet plan suggested by the nutritionist and refrain from doing exercise activities other than the scheduled ones. The intervention groups participated in an orientation session one week before the first training session to familiarize themselves with the exercises. The training program was implemented three times a week for eight weeks. Hemoglobin, hematocrit, fibrinogen, and ESR variables were measured in all three groups before and after the trial. The control group subjects were visited weekly by a cardiologist, and the training groups performed aerobic and combined exercise according to protocol in the eight weeks of the study. The control group did not have regular exercise activity for more than 30 minutes a day.
Subjects in the aerobic and combined exercise groups participated in an eight-week training program based on the recommendations of the American College of Sports Medicine (13).
Aerobic exercise program: Participants performed Aerobic training exercises three days a week for eight weeks. The exercise included treadmill walking (House Fit, Taiwan) (20 to 30 minutes), pedaling on a fixed bike (TA Sport, Taiwan) (10 to 12 minutes), and using an arm ergometer (Fitness Emotion, Taiwan) (8 to 10 minutes). Stretching exercises were used for warm-up at the beginning of the session and for cooling down at the end of the exercise session (5 to 10 minutes). Exercises began with moderate intensity. In addition to the amount of fatigue and cardiac symptoms, the duration and intensity of exercise were adjusted at 60% of the maximum heart rate. The intensity and duration of exercise gradually increased based on patients' ability to reach 80% of their maximum heart rate in the last two sessions (13, 14).
Combined exercise program: In each session of combined training exercise, the patients first performed aerobic and simultaneous resistance training after a short rest, as described below. Resistance training consisted of exercises performed three sessions a week for eight weeks. The resistance training program included three sets of physioball squats, shoulder flexion, hip flexion, shoulder abduction, hip abduction, elbow flexion, plantar flexion, and ankle dorsiflexion with 8-15 repetitions. The activities were initially performed with eight repetitions using a weak thera-band (yellow). Then, two repetitions were added to each activity in every session until 15 repetitions were reached. The gradual increase of repetitions continued after increasing the strength of the thera-band (pink) (15, 16).
3.1. Measurement of Blood Factors
Blood sampling was performed in two stages: 48 hours before the first training session and 48 hours after the last training session. Then, 10 cc of blood Sampling was collected from the peripheral brachial vein (between 8 and 9 a.m.) and put in experimental tubes. In the laboratory of Imam Ali (AS) Hospital, Measurement of Hemoglobin and Hematocrit levels was performed using the automated blood cell analyzer (Sysmex, Kobe, Japan) – Fibrinogen (STAGO, France) and ESR (Sadiman Reader machine, Iran).
Descriptive statistics, including mean and Standard Deviation (SD), were used to describe data. The normality of data distribution was examined using the Shapiro-Wilk test. Parametric paired sample t-test and one-way ANOVA were used to investigate intragroup and intergroup differences in SPSS v.21 software at a significance level of P ≤ 0.05.
4. Results
Demographic characteristics of the subjects in different groups are shown in Table 1. There was no significant difference between the mean age (P = 0.608), height (P = 0.324), weight (P = 0.841), and body mass index (BMI) (P = 0.774) among the three aerobic, combined, and control groups (Table 1).
| Variables | Aerobic Exercises | Combined Exercises | Control | Total | P-Value |
|---|---|---|---|---|---|
| Age (y) | 59.20 ± 5.80 | 57.40 ± 6.80 | 56.30 ± 5.83 | 57.63 ± 6.38 | 0.608 |
| Height (cm) | 172.70 ± 5.81 | 168.80 ± 6.92 | 172.40 ± 6.20 | 171.30 ± 6.31 | 0.324 |
| Weight (kg) | 79.95 ± 11.60 | 77.00 ± 10.91 | 77.50 ± 12.58 | 78.15 ± 11.70 | 0.841 |
| BMI (Kg/m2) | 26.76 ± 3.36 | 27.10 ± 4.20 | 25.98 ± 3.25 | 26.61 ± 3.60 | 0.774 |
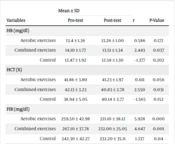
The results of the paired sample t-test showed a significant difference (decrease) in hemoglobin (P = 0.037) and hematocrit (P = 0.031) before and after the combined exercises. However, no significant difference was observed in the aerobic group and the control group (P > 0.05) (Table 2). The one-way ANOVA test did not show a significant difference in the hemoglobin (P = 0.487) and hematocrit (P = 0.306) indices before and after the intervention (Table 3).
| Variables | Mean ± SD | t | P-Value | |
|---|---|---|---|---|
| Pre-test | Post-test | |||
| HB (mg/dL) | ||||
| Aerobic exercises | 13.4 ± 1.36 | 13.26 ± 1.00 | 0.586 | 0.172 |
| Combined exercises | 14.10 ± 1.77 | 13.51 ± 1.34 | 2.443 | 0.037 |
| Control | 12.47 ± 1.92 | 12.58 ± 1.30 | - 1.377 | 0.202 |
| HCT (%) | ||||
| Aerobic exercises | 41.86 ± 3.80 | 41.23 ± 1.97 | 0.611 | 0.056 |
| Combined exercises | 42.13 ± 3.23 | 40.83 ± 2.78 | 2.550 | 0.031 |
| Control | 38.94 ± 5.05 | 40.14 ± 3.77 | - 1.565 | 0.152 |
| FIB (mg/dL) | ||||
| Aerobic exercises | 259.50 ± 42.98 | 221.10 ± 38.12 | 5.928 | 0.000 |
| Combined exercises | 267.10 ± 37.78 | 232.00 ± 25.05 | 4.647 | 0.001 |
| Control | 242.30 ± 42.27 | 232.20 ± 35.11 | 1.337 | 0.114 |
| ESR (mm/hr) | ||||
| Aerobic exercises | 16.90 ± 6.02 | 8.90 ± 2.92 | 5.689 | 0.000 |
| Combined exercises | 16.00 ± 7.42 | 9.00 ± 4.52 | 5.653 | 0.000 |
| Control | 18.00 ± 5.65 | 11.60 ± 3.37 | 6.532 | 0.000 |
The following paired sample t-test showed a significant difference in the fibrinogen level in aerobic (P = 0.000) and combined exercises (P = 0.001) groups before and after the intervention. Nevertheless, no significant difference was observed in the control group (P = 0.114). The results in the ESR variable revealed a significant difference in all three groups of the present study (P = 0.000) (Table 2), and the One-way ANOVA test did not show a significant difference in fibrinogen (P = 0.197) and ESR (P = 0.104) variables in the three groups (Table 3).
| Variables | Sum of Squares | df | Mean Square | F | P-Value |
|---|---|---|---|---|---|
| HB (mg/dL) | 0.487 | ||||
| Between groups | 2.221 | 2 | 1.110 | 0.738 | |
| Within groups | 40.618 | 27 | 1.504 | ||
| Total | 42.839 | 29 | - | ||
| HCT (%) | 0.306 | ||||
| Between groups | 6.081 | 2 | 3.040 | 0.352 | |
| Within groups | 233.066 | 27 | 8.632 | ||
| Total | 239.147 | 29 | - | ||
| FIB (mg/dL) | 0.197 | ||||
| Between groups | 867.867 | 2 | 403.433 | 0.365 | |
| Within groups | 29834.500 | 27 | 1104.981 | ||
| Total | 30641.367 | 29 | - | ||
| ESR (mm/hr) | 0.104 | ||||
| Between groups | 46.867 | 2 | 23.433 | 1.742 | |
| Within groups | 363.300 | 27 | 13.456 | ||
| Total | 410.167 | 29 | - |
5. Discussion
This study showed that combined exercises caused significant decreases in hemoglobin and hematocrit. Both aerobic and combined exercises showed significant reductions in fibrinogen and ESR. No significant difference was found between the effects of aerobic and combined exercise on hemorheological indicators. The results were consistent with similar studies. Çiçek reported that resistance training significantly reduced hemoglobin and hematocrit levels, while aerobic training significantly affected (17). Mehrfazari et al. and Amiri Parsa et al. found that eight weeks of aerobic exercise did not have a significant effect on hemoglobin and hematocrit levels (11, 18). In addition, aerobic exercise had a different impact than combined exercise and intergroup effects. Hematocrit levels indicate blood viscosity and contribute to cardiovascular disease. Exercise leads to physiological changes and lowers risk factors, benefiting cardiovascular health (19, 20). Exercise improves hematological indicators, including hematocrit, and reduces blood viscosity, which enhances red blood cell efficiency and increases blood flow (4, 19). Some studies contradict the present findings. Pabisiak et al. found that aerobic exercise decreased hemoglobin and hematocrit levels (7), but Sheikholeslami et al. found no significant decrease in these levels after six weeks of resistance training (21). Heidari et al.'s study found significant improvements in hematocrit, hemoglobin, and red blood cell count after eight weeks of aerobic training (22). Exercise factors (age, gender, fitness level, type, intensity, and duration) can impact CABG patients' results (23). Countries recommend different methods (24). Aerobic and combined exercise groups showed significant fibrinogen reduction, consistent with prior studies (25-27). Sackett et al. found that eight weeks of high-intensity training reduced fibrinogen levels (25). Ahmadizad et al. observed a decrease in fibrinogen levels in both types of exercise without significant difference (26). Mirsaiedi indicated a significant reduction in the fibrinogen level after eight weeks of combined training (27). However, these results were inconsistent with those of other studies. In Amiri Parsa, the reduction in fibrinogen after eight weeks of exercise training was insignificant (18). Additionally, Sobhani et al. showed that exercise training had increased blood fibrinogen levels in 30 patients with a history of coronary artery surgery (28). Fibrinogen is the best coagulation indicator for assessing cardiovascular problems (27), and its increased blood circulation is a risk factor for cardiovascular diseases. Factors such as fibrinogenolysis, conversion to fibrin, transfer to interstitial space, slower production during exercise, and data correction cause reduced fibrinogen plasma levels after endurance activity (25, 27). Erythrocyte sedimentation rate decreased significantly, consistent with Avazpour et al. (29). Other studies, such as Pabisiak et al. and Sandor et al., also reported decreased ESR after sports activities (7, 8). Fathi et al. found no significant reduction in ESR after aerobic exercise (12), which is consistent with a systematic review by Harpham et al. (30). However, the present study yielded different results. Erythrocyte sedimentation rate levels depended on pro-inflammatory products and red blood cell shape (31). Inflammation could increase ESR and accelerate atherosclerosis plaque formation, causing plasma proteins to stick to red cells (32, 33). Sports activities could reduce ESR by reducing the adhesion of plasma proteins to red blood cells (34, 35).
5.1. Limitations
Despite the explanations, one of the study's main limitations was the patients' fear of doing exercise training in the initial sessions. The inclusion and exclusion criteria were also part of the limitations.
5.2. Conclusions
The present study showed improved hemorheological indicators (hemoglobin, hematocrit, fibrinogen, and ESR) in middle-aged male patients undergoing CABG after eight weeks of aerobic and combined exercise. However, the effect of aerobic exercise on hemoglobin and hematocrit was insignificant. There was no significant difference between the effect of aerobic and combined exercise on hemorheological indices. Aerobic and combined exercises were effective intervention methods to improve patients' hemorheological indicators and conditions after CABG. Further studies are needed to generalize the results to other statistical communities.