1. Background
Hair manifests youth and health psychologically and based on interpersonal relationships. Hair loss has an adverse effect on a person's social status, self-confidence, and psychological dimension (1, 2). Androgenic alopecia (AGA) is an androgen-dependent hereditary disorder, the progressive thinning of hair with various patterns. The pattern of hair loss in women is seen centrally (based on Ludwig's classification) without temporal-frontal or whole-head progression. However, in men, bilateral hair loss in the temporal region and vertex region (based on Hamilton's classification) is the predominant pattern (3, 4).
AGA is characterized by hair follicle shrinkage manifesting as hair loss due to systemic androgens and genetic factors (4). Most changes are in the third and fourth decades of life, but hair loss begins immediately after puberty and continues gradually (5). AGA is the most common cause of hair loss in men and women (6).
Female pattern hair loss (FPHL) has replaced AGA in women due to the unclear relationship between androgens and their incidence (7). FPHL is the most common hair loss disorder in women. Initial symptoms may appear in the teenage years, leading to progressive and extensive hair loss with a specific pattern (8).
Clinical evaluation in individuals with AGA should include examination of the scalp, facial hair, body, and all skin and nails. Although examining the scalp is usually normal in AGA, seborrheic dermatitis (SD) can be considered a worsening factor of AGA (9). When AGA is chronic, the scalp becomes atrophic, and contact with sunlight can aggravate AGA (3, 10, 11).
SD has a wide range of mild (in the form of dandruff), moderate (in the form of redness and scaling), and severe (in the form of secondary infections), and its initial symptoms are redness and peeling around the hair follicle (12, 13).
SD has a wide range of mild (in the form of dandruff), moderate (in the form of redness and scaling), and severe (in the form of secondary infections), and initial symptoms are redness and peeling around the hair follicle (12, 13). Although hair loss is not always related to SD, some studies state that hair loss occasionally occurs with increased telogens in people with chronic SD, which will be reversible when the inflammation is removed (14, 15). Follicular involvement in SD with extensive and progressive hair loss has not been mentioned in studies (16). Men are affected more often than women (3.0% vs. 2.6%) in all age groups, suggesting that SD may be associated with sex hormones such as androgens (12-14).
Since androgenetic alopecia is affected by race, genetics, and geographical conditions, it is necessary to investigate the prevalence of these two diseases together in the Iranian population. When these two complications are accompanied by each other, a more appropriate treatment process should be followed because their treatment affects each other.
2. Objectives
When SD and AGA appear in the same person, appropriate and timely treatment for those cases is essential. Therefore, the main aim of the present study was to evaluate the association of SD with AGA in patients referred to the skin clinic located at Rasoul Akram Hospital in Tehran in 2021.
3. Methods
This descriptive-analytical study was conducted on patients with AGA referred to the Dermatology Clinic of Rasoul Akram Hospital in Tehran, Iran. Sampling was done sequentially and stopped when the number of samples reached the required size. The relevant sample size equation was used to determine the sample size, which is estimated to be 200 patients. Considering the possible 20% dropout of patients, the sample size was finally increased to 250. The number of effective parameters in determining the sample size for the present study was received from Kibar et al. (15).
Inclusion criteria were a clinical diagnosis of AGA, as the predominant diagnosis by a dermatologist, and an age between 18 and 60 years. Exclusion criteria included receiving previous treatment for hair loss, previous treatment for neurological disease, a history of immunodeficiency disease, and an unwillingness to participate in the study.
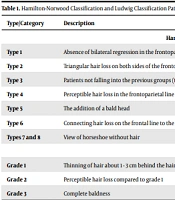
Once informed consent has been obtained from the patients, the required information of the patients is obtained, such as their age, gender, occupation, marital status, skin, hormonal or systemic diseases, medications taken by the patient, smoking and alcohol consumption, and family history of hair loss. Skin color and face and skin type were recorded by a researcher-made checklist. Then, the patients were examined regarding SD, and the presence or absence of seborrheic dermatitis and disease symptoms, including erythema, dandruff, and the spread of the lesion, were checked. In addition, the severity of dermatitis was examined, and its classification includes "no involvement"="level 0", involvement of less than half of the head="level 1", involvement of half of the head="level 2" and involvement in more than half of the head=" level 3" (12-14). In the following, information about AGA includes the location of hair loss on the head, the male pattern hair loss according to the Hamilton-Norwood classification (HNC) (Table 1). The female pattern hair loss was determined according to the Ludwig classification pattern (LC) (Table 1) (15).
| Type/Category | Description |
|---|---|
| Hamilton-Norwood Classification | |
| Type 1 | Absence of bilateral regression in the frontoparietal line |
| Type 2 | Triangular hair loss on both sides of the frontoparietal line |
| Type 3 | Patients not falling into the previous groups (types 1 and 2) have non-symmetrical hair loss in the frontoparietal line. |
| Type 4 | Perceptible hair loss in the frontoparietal line is known as baldness. |
| Type 5 | The addition of a bald head |
| Type 6 | Connecting hair loss on the frontal line to the frontoparietal line |
| Types 7 and 8 | View of horseshoe without hair |
| Ludwig Classification | |
| Grade 1 | Thinning of hair about 1 - 3 cm behind the hairline |
| Grade 2 | Perceptible hair loss compared to grade 1 |
| Grade 3 | Complete baldness |
Finally, the collected data were analyzed using SPSS software Version 24 and relevant statistical tests at a significance level of α = 0.05. The descriptive results obtained for quantitative variables were expressed as mean and standard deviation and for categorical qualitative variables as frequency and frequency percentage. Kolmogorov–Smirnov test was used to evaluate the normal distribution in different parameters. The Paired sample t-test was utilized to compare the quantitative variables in two different modes, but the Wilcoxon test was used when the distribution was abnormal. The Chi-squared test was used to compare the two qualitative variables. The Mann–Whitney U test was used for two quality and quantitative variables, and the Pearson correlation coefficient (r) test was applied to compare two quantitative variables.
4. Results
The average age was 35.64 ± 8.11; 146 people (58.6%) were 36 to 60 years old, and 104 (41.4%) were 18 to 35. The results showed that 169 (67.2%) women and 81 (32.8%) men participated in the study. The most common occupation of the patients was housewife, which was observed in 78 people (31%), and 164 people (65.5%) were married. According to the findings, 47 people (19%) had a history of hormonal diseases, 43 people (17.2%) had a history of skin diseases, 56 people (22.4%) had a history of systemic diseases, and 194 people (77.6%) had a history of hair loss in the family. According to the answers of the participants, 65 people (25.9%) were taking medicine, and 73 people (29.3%) had a history of smoking and alcohol consumption. The most common type of skin related to oily skin was observed in 112 (48.3%) patients. Further, the most significant number of patients, 138 (55.2%), were white, and the most alopecia was in the frontal area in 164 (65.5%) patients. The study showed that only 78 people (31%) had erythema and dandruff, and 142 people (56.9%) had lesions below 1%.
About 142 out of 250 (56.9%) patients had SD, and the highest severity of SD was grade 1 (involvement of less than half of the head) in 116 out of 250 people (46.6%).
The frequency of classification of hair loss patterns for men and women is presented in Table 2. Based on the findings mentioned above, 90 people were classified in the Hamilton-Norwood classification (HNC) and 164 in the Ludwig classification (LC) (Table 2).
| Type/Category | No. (%) | |||
|---|---|---|---|---|
| Hamilton-Norwood Classification (HNC) of Male Pattern Hair Loss | ||||
| Type 1 | 14 (15.6) | |||
| Type 2 | 27 (30) | |||
| Type 3 | 23 (25.6) | |||
| Type 4 | 6 (6.6) | |||
| Type 5 | 10 (11.1) | |||
| Type 6 | 10 (11.1) | |||
| Total | 90 (100) | |||
| Ludwig Classification (LC) of Female Pattern Hair Loss | ||||
| Grade 1 | 142 (86.6) | |||
| Grade 2 | 22 (13.4) | |||
| Total | 164 (100) | |||
The results of the relationship between the severity of SD and the study variables, including age, gender, history of drugs, smoking, and alcohol consumption, history of previous diseases, history of hair loss in the family, skin color, skin type, and location of facial involvement are shown in Table 3. The results of the analysis using the chi-squared test showed a significant relationship only between the severity of SD and "history of hair loss in the family" (P < 0.05), while no significant correlation between the severity of SD and other variables (P > 0.05).
| Variables | Severity of Seborrheic Dermatitis | P-Value | |||
|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | ||
| Age | 0.09 | ||||
| 18 - 35 | 47 | 34 | 13 | 9 | |
| 90 - 36 | 61 | 82 | 4 | 0 | |
| Sex | 0.106 | ||||
| Male | 39 | 26 | 9 | 9 | |
| Female | 69 | 90 | 9 | 0 | |
| History of drug use | 26 | 35 | 4 | 0 | 0.81 |
| History of smoking and alcohol consumption | 26 | 39 | 0 | 6 | 0.071 |
| History of skin disease | 22 | 17 | 4 | 0 | 0.8 |
| History of hormonal disease | 17 | 26 | 4 | 0 | 0.82 |
| History of systemic disease | 22 | 34 | 0 | 0 | 0.45 |
| Family history of hair loss | 91 | 86 | 17 | 0 | 0.031 |
| Facial skin color | 0.52 | ||||
| White | 56 | 73 | 4 | 4 | |
| Wheat color | 52 | 43 | 13 | 4 | |
| Skin type | 0.38 | ||||
| Greasy | 56 | 47 | 13 | 4 | |
| Dry | 9 | 26 | 4 | 4 | |
| Normal | 24 | 19 | 0 | 0 | |
| Scalp involvement | |||||
| Frontal | 73 | 82 | 9 | 0 | 0.2 |
| Temporal | 35 | 30 | 4 | 9 | 0.18 |
| Parietal | 56 | 52 | 9 | 0 | 0.55 |
| Occipital | 39 | 22 | 9 | 0 | 0.28 |
The relationship between the severity of SD and the pattern of AGA in women and men showed that based on the pattern of AGA in men, the highest severity of SD (3rd degree=involvement of more than half of the head) corresponds to the pattern of type “2” hair loss (triangular hair loss in both sides of the front parietal line) in four patients. In addition, this result was observed in women with 2nd-degree dermatitis (involvement of half of the head) related to type 1 hair loss pattern (thinning of the head about 1 - 3 cm behind the hairline) in nine patients. Further, there was a significant relationship between the parameters of the AGA pattern in men and the severity of SD (P < 0.05) (Table 4).
| Variable and Type/Category | Severity of Seborrheic Dermatitis | P-Value | |||
|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | ||
| Hamilton-Norwood classification (HNC) of male pattern hair loss | 0.021 | ||||
| Type 1 | 4 | 0 | 4 | 0 | |
| Type 2 | 13 | 9 | 0 | 4 | |
| Type 3 | 9 | 13 | 0 | 0 | |
| Type 4 | 0 | 4 | 0 | 0 | |
| Type 5 | 5 | 0 | 5 | 0 | |
| Type 6 | 9 | 0 | 0 | 0 | |
| Ludwig classification (LC) of female pattern hair loss | 0.42 | ||||
| Grade 1 | 56 | 77 | 9 | 0 | |
| Grade 2 | 13 | 9 | 0 | 0 | |
5. Discussion
The results of the present study showed that 142 out of 250 patients (56.9%) had seborrheic dermatitis (SD). The highest severity of SD was grade “1”, observed in almost half of the patients. The severity of SD with involvement of more than half of the head (grade “3”) was only in the age group of 18 to 35 years and men. However, there was no significant relationship between the severity of SD and age and sex parameters. Based on the androgenic alopecia (AGA) pattern in men, the highest severity of SD is related to the pattern of hair loss type "2" (triangular hair loss on both sides of the frontoparietal line). The highest severity of SD In women was grade "2", which is observed in the pattern of hair loss type "1" (thinning of hair about 1 - 3 cm behind the hairline). Only the parameter of the AGA pattern in men had a significant relationship with the severity of SD. In a similar study by Sarlak et al., the level of seborrheic dermatitis was evaluated in 60 out of 160 patients (37.5%) (17). Moreover, in another study by Jang et al., the most common pattern of AGA in men was HNC-3 grade and LC-1 grade in women. SD was the most common comorbidity in affected women and men (18). In the study by Ummiti et al., 66 men and 25 women with AGA were included. The most common finding was the heterogeneity of hair shaft thickness in all male and female patients (19). Hu et al. included 750 men and 200 women as the patient group (case) and 100 men and 50 women as the healthy group (control). The mentioned study showed that the heterogeneity of hair shaft thickness was observed in more than 20% of men (20). Generally, the results of previous similar studies, in line with the results of the present study, have emphasized that higher degrees of hair loss due to thinner and more vulnerable form are prone to the occurrence of severe dermatitis and the hair of these patients should be strengthened in a better way.
Another important finding of the present study was that half of the patients had oily skin, and most were white. The most common location of alopecia was the frontal area of the head in 164 patients (65.5%). In addition, the most pattern of male hair loss is related to type 2-HNC (triangular hair loss on both sides of the frontoparietal line) in 27 patients (10.7%), and the most pattern of female hair loss is related to grade "1" (thinning of hair about 1 - 3 cm behind the hairline) in 149 (56.9%) patients. The highest intensity of dermatitis was related to grade 1-LC in people with white and wheatish skin color. Based on the skin type, only in dry and oily skins was the highest intensity of SD, i.e., intensity of grade “3”. The highest severity of SD, i.e., grade “3”, also occurred in the temporal region. The present study showed no meaningful relationship between facial skin color, skin type, and scalp involvement parameters. Kibar et al. observed that multi-hair follicular and honeycomb pigmentation is probably correlated with androgenetic hair loss (15). Ummiti et al. found that honeycomb pigmentation was observed in the scalp of most patients, but it was not related to the severity of hair loss (19). Hu et al. reported that yellow spots, white spots, and scalp pigmentation were associated with the severity of hair loss (20). Therefore, the results of previous similar studies and the current research confirm that the presence of different skin pigments and dryness or having too much fat on the skin causes hair loss to become more severe and dermatitis to appear more severe. Therefore, solving these problems can prevent dermatitis or its progression (if it occurs).
5.1. Limitations
Several study participants were unfamiliar with the specialized terms related to dermatitis and hair loss, which was a critical limitation of the present study. This limitation in the time of questioning by the researcher caused problems in recording the correct and accurate answers. The questioner tried to familiarize the patients with specialized terms by providing appropriate explanations so that the patients could give the most accurate and realistic answers.
5.2. Conclusions
Considering the relationship between the severity of seborrheic dermatitis and male hair loss patterns, doctors and experts on skin diseases should evaluate this issue with more care and follow-up. Removing dandruff symptoms can help improve hair loss. Based on the results, evaluating a person's disease history and family history is critical in determining the pattern of hair loss, preventing the progression of this problem, and on-time treatment.