1. Background
Patients receiving long-term hemodialysis (HD) treatment are more susceptible to infections, leading to a higher likelihood of mortality and morbidity (1, 2). Sepsis is the second most common cause of death in individuals on chronic HD following cardiovascular events (3). Catheter-related infections are the most common causes of bacteremia in patients undergoing HD (4). Risk factors for infection emergence in various studies include patient age, duration of access, presence of underlying diseases such as diabetes mellitus and an immune-compromised state, catheter insertion site, type of access, history of previous access-related infection, and nasal colonization with Staphylococcus aureus (S. aureus) (5-7). Hypoalbuminemia has been described as one of the risk factors with an increased risk of bacteremia, infection, and worse prognosis in HD patients. (5, 8, 9). Short-term mortality and hospitalization are three times higher in patients with low serum albumin levels than those with normal serum albumin levels (10). There are various mechanisms by which HD patients develop hypoalbuminemia: Decreased protein synthesis due to malnutrition, changes in the volume of distribution, loss of protein during dialysis, which is common in patients on intermittent HD, and decreased synthesis of anabolic hormones, which are involved in albumin production, such as thyroid hormones and cortisol. On the other hand, albumin is also considered a negative acute phase protein, and its synthesis is reduced in acute conditions and inflammation (11).
2. Objectives
Identifying the risk factors associated with hypoalbuminemia may help to prevent its incidence and infection rates in HD patients. In this study, the prevalence of hypoalbuminemia was investigated in patients undergoing chronic HD and its correlation with catheter-related infections. We also studied the bacteria most frequently associated with catheter-related infections.
3. Methods
3.1. Study Design and Sampling
This retrospective cross-sectional study evaluated the association between serum albumin level and catheter-related infection in chronic HD patients. This study included all individuals with end-stage kidney disease (ESKD) who were receiving routine HD with a venous catheter and were admitted to the hospital due to clinical signs and symptoms or laboratory evidence indicating a possible catheter-related infection. The patients were recruited from Hasheminejad Kidney Center and Firoozgar General Hospital in Tehran from June 2018 to July 2019. The study included patients with ESKD who were undergoing chronic HD treatment. The inclusion criteria were at least 18 years old, local or systemic signs of infection such as erythema and discharge from the catheter exit site (exit site infection), tenderness of the subcutaneous tunnel (tunnel infection), and fever and chills or catheter-related bloodstream infection (CRBSI) which means they had positive blood and catheter lumen cultures (both with the identical organism), did not have another source of infection, and did not have an immune-compromising condition such as HIV/AIDS or malignancies. The control group was selected from patients with ESKD on routine HD from the same centers who had not experienced any episode of catheter-related infection. All patients had a double-lumen tunneled catheter, and the aseptic placement technique was observed in all cases, according to the surgical team reports. Patients who did not meet the mentioned criteria according to the microbial culture results were excluded from the study.
Serum albumin and other laboratory parameters such as hemoglobin (Hgb), serum creatinine (Cr), phosphorus (P), potassium (K), parathyroid hormone (PTH), vitamin D, random blood sugar (BS), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were also checked as surrogate markers of inflammation.
3.2. Statistical Analysis
Continuous variables were described as mean ± SD (median/Interquartile range), and categorical variables were expressed as frequency/percent. The Kolmogorov-Smirnov test was used to evaluate the variables' normality. An independent samples t-test (Mann-Whitney U test for nonparametric variables) was used to compare continuous variables between the two groups. A one-way ANOVA test assessed the difference between constant variables among more than two groups. The chi-square test was used to compare two categorical variables. A P-value less than 0.05 was assumed to be statistically significant.
4. Results
A total of 420 patients with ESKD on routine HD were enrolled in the study, 210 of which belonged to the target group who experienced at least one episode of catheter-related infection, and the other 210 participants belonged to the control group without any episode of catheter-related infection. The mean age was 56 ± 24 in the target group and 55.6 ± 18 in the control group. There was no significant difference in the mean age between the two groups (P = 0.8). There was no significant difference in the demographic composition of the two groups (P = 0.3). The basic characteristics of the patients and the results of laboratory tests are shown in Table 1. The levels of ESR and CRP were much higher in the target group than in the control group (P = 0.002 and P = 0.001, respectively). There was a significant difference in the K levels between the target and the control groups (P = 0.01). The prevalence of hyperkalemia was higher in the target group, and the prevalence of normal K levels was higher in the control group. The prevalence of hyperphosphatemia was higher in the target group, while most patients in the control group had low or normal serum phosphate levels (P < 0.001). The differences between Hgb, 25-OH vitamin D, and PTH levels in the case and control groups were not found to be significant.
| Variables | Case | Control | P-Value |
|---|---|---|---|
| Hgb (g/dL) | 10.2 ± 2.9 | 10.8 ± 7.3 | 0.6 |
| ESR (cm) | 55 ± 45.7 | 43.4 ± 30.2 | 0.002 |
| CRP (mg/dL)median (IQR) | 38 (31.3) | 25.9 (32.2) | 0.001 |
| Gender | 0.3 | ||
| Male | 125 (59.5) | 135 (64.3) | |
| Female | 85 (40.5) | 75 (35.7) | |
| Age (y) | 0.1 | ||
| ≤ 50 | 67 (31.9) | 83 (39.5) | |
| > 50 | 143 (68.1) | 127 (60.5) | |
| K (mEq/L) | 0.01 | ||
| Hypokalemia | 10 (4.8) | 6 (2.9) | |
| Normokalemia | 120 (57.1) | 148 (70.8) | |
| Hyperkalemia | 80 (38.1) | 55 (26.3) | |
| Vitamin D (ng/dL) | 0.5 | ||
| Deficient | 83 (39.5) | 104 (51.2) | |
| Insufficient | 38 (18.1) | 41 (20.2) | |
| Sufficient | 60 (28.6) | 58 (28.6) | |
| P (mg/dL) | < 0.001 | ||
| Hypophosphatemia | 17 (8.2) | 56 (26.7) | |
| Normal | 54 (26) | 81 (38.6) | |
| Hyperphosphatemia | 137 (65.9) | 73 (34.8) | |
| PTH (pg./mL) | 0.3 | ||
| Hypoparathyroidism | - | 2 (1) | |
| Normal | 29 (13.8) | 37 (18.1) | |
| Hyperparathyroidism | 156 (84.3) | 165 (80.9) | |
| Random BS (mg/dL) | 0.1 | ||
| Hypoglycemia | 5 (2.4) | 55 (26.3) | |
| Normoglycemia | 55 (26.3) | 70 (33.7) | |
| Hyperglycemia | 149 (71.3) | 131 (63) | |
| Albumin (g/L) | 0.2 | ||
| Low | 90 (42.9) | 88 (41.9) | |
| Normal | 120 (57.1) | 122 (58.1) |
Abbreviations: Hgb, hemoglobin; P, phosphorus; K, potassium; BS, random blood sugar; PTH, parathyroid hormone.
a Values are expressed as No. (%) or mean ± SD unless otherwise indicated.
b Potassium 3.5 - 5.2 (mEq/L), vitamin D 20 - 40 ng/dL, phosphorus 3.5 - 5.5 mg/dL, parathyroid hormone (PTH) 10 - 60 pg/mL.
Serum albumin levels were low in 42.9% of the target and 41.9% of the control group. There was no difference in mean albumin level between the case and the control groups (P = 0.2). In addition, no difference was observed in albumin levels between the case and the control groups in different age or sex groups (P = 0.4 and P = 0.7) and (0.2 and 0.9), respectively (Table 2).
The mean serum albumin level was higher in patients with hyperphosphatemia in the control group (P = 0.03), but in patients with normal or low phosphate levels, there was no difference in albumin level between the case and the control group. The median albumin level was significantly higher in patients of the control group with normal PTH levels than in the target group (P = 0.03).
Hypoglycemic patients in the target group had lower albumin levels than those in the control group (P = 0.03).
| Variables | Albumin | P-Value | |
|---|---|---|---|
| Case | Control | ||
| Age | |||
| ≤ 50 | 3.6 ± 0.5 | 3.7 (0.7) | 0.4 |
| > 50 | 3.4 ± 0.5 | 3.5 (0.9) | 0.7 |
| Gender | |||
| Male | 3.5 ± 0.5 | 3.6 (0.8) | 0.2 |
| Female | 3.5 ± 0.5 | 3.6 (0.8) | 0.9 |
| Serum K | |||
| Low | 2.9 ± 0.8 | 3.5 (1.5) | 0.3 |
| Normal | 3.5 ± 0.5 | 3.6 (0.7) | 0.4 |
| High | 3.6 ± 0.5 | 3.6 (1) | 0.8 |
| Vitamin D | |||
| Deficiency | 3.4 ± 0.6 | 3.5 (0.8) | 0.6 |
| Insufficiency | 3.5 ± 0.5 | 3.5 (0.9) | 0.6 |
| Normal | 3.6 ± 0.5 | 3.7 (0.8) | 0.5 |
| Serum P | |||
| Low | 3.5 ± 0.7 | 3.4 (0.5) | 0.5 |
| Normal | 3.6 ± 0.6 | 3.4 (0.5) | 0.1 |
| High | 3.5 ± 0.8 | 3.6 (0.5) | 0.03 |
| Serum PTH level | |||
| Low | 3.9 ± 1.9 | - | 0.5 |
| Normal | 3.3 ± 0.6 | 3.7 (0.7) | 0.03 |
| High | 3.5 ± 0.5 | 3.6 (0.8) | 0.07 |
| Random BS | |||
| Hypoglycemia | 3.3 ± 0.4 | 3.9 (0.7) | 0.03 |
| Normoglycemia | 3.5 ± 0.5 | 3.5 (0.9) | 0.3 |
| Hyperglycemia | 3.5 ± 0.51 | 3.6 (0.7) | 0.1 |
| Total Albumin | 3.5 ± 0.5 | 3.6 (0.7) | 0.2 |
Abbreviations: PTH, parathyroid hormone; P, phosphorus; K, potassium; BS, random blood sugar.
a Values are expressed as mean ± SD or median (IQR).
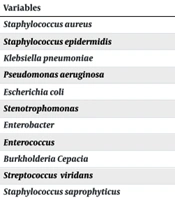
Staphylococcus aureus was the most common bacteria (38.6%), and S. epidermidis was the second most common microorganism (27.6%) detected from the target group patients' blood and catheter lumen cultures. The lowest albumin mean was associated with Stentrophomonas (3.2 ± 0.7), and the highest albumin mean was associated with Enterococcus (3.9 ± 0.3) (Table 3). However, there was no significant difference in mean albumin levels between the infections caused by different microorganisms (P = 0.8).
| Variables | No. (%) | Albumin; Mean ± SD |
|---|---|---|
| Staphylococcus aureus | 81 (38.6) | 3.4 ± 0.5 |
| Staphylococcus epidermidis | 58 (27.6) | 3.5 ± 0.5 |
| Klebsiella pneumoniae | 21 (10) | 3.6 ± 0.4 |
| Pseudomonas aeruginosa | 20 (9.5) | 3.6 ± 0.5 |
| Escherichia coli | 14 (6.7) | 3.4 ± 0.6 |
| Stenotrophomonas | 6 (2.9) | 3.2 ± 0.7 |
| Enterobacter | 4 (1.9) | 3.7 ± 0.3 |
| Enterococcus | 2 (1) | 3.9 ± 0.3 |
| BurkholderiaCepacia | 2 (1) | 3.5 ± 0.6 |
| Streptococcus viridans | 1 (0.5) | - |
| Staphylococcus saprophyticus | 1 (0.5) | - |
5. Discussion
This study investigated 420 patients, with 210 individuals in the case group and 210 in the control group. There was no difference in age or gender between the two groups. The case group revealed significantly higher levels of potassium (P = 0.01), ESR (P = 0.002), CRP (P = 0.001), and phosphate (P = 0.001). Both groups had a high prevalence of vitamin D deficiency and insufficiency. No significant difference was found in albumin levels between the two groups (P = 0.2). The most common bacteria responsible for catheter-related infections were found to be S. aureus (38.6%), S. epidermidis (27.6%), Klebsiella (10%), and Pseudomonas aeruginosa (9.5%). Notably, the lowest albumin levels were seen in sepsis with Stentrophomonas, S. aureus, and E. coli. Still, there was no significant difference in the albumin level among different bacterial infections (P = 0.8).
In Bohlke et al., gram-positive cocci were the most common microorganisms isolated from catheter-related bacteremia. According to US data, coagulase-negative staphylococci (CoNS) are found in 32% to 45% of cases, S. aureus in 22 to 29% and Enterococci in 9% to 13% of cases (12), which is lower than the prevalence in the present study. In Suzuki et al., gram-positive cocci were reported to be responsible for half to three-fourths of the catheter-related infection cases (13). The difference between microorganisms could be different from hospital to hospital.
In Powe et al., 11.7% of 4005 HD patients and 9.4% of 913 peritoneal dialysis (PD) patients had at least one episode of septicemia over seven years of follow-up. Low serum albumin at baseline was also an identifiable risk factor for septicemia among HD patients. This study revealed that a decreased level of serum albumin in dialysis patients may suggest malnutrition and has previously been demonstrated to be a powerful predictor of mortality. The results indicated that the risk of septicemia in individuals undergoing dialysis could be reduced by implementing nutritional counseling, avoiding low-protein diets several months before starting renal replacement therapy, and increasing the dialysis dose to improve appetite and correct acidosis. These interventions aim to prevent and treat dialysis-associated malnutrition in this vulnerable population (14).
No significant difference was observed in serum albumin levels between the case and the control groups. The finding contradicts the results of prior studies that demonstrated an association between hypoalbuminemia, bacteremia, and sepsis (14-17). In Lata et al., hypoalbuminemia was a prognostic factor in catheter-related infection rather than a risk factor (2). In addition, in the study of Tanriover et al., hypoalbuminemia was a risk factor for recurrent catheter infections in patients with a positive history of previous catheter-related infection (18). In the study that retrospectively evaluated the outcomes of patients with catheter-associated bacteremia over two years, the risk factors for bacteremia were recent catheter change, previous bloodstream infections, diabetes mellitus, and poor hygiene (18). Although no significant association was found between serum albumin levels and catheter-related infections, these disparities do not invalidate the conclusions established by earlier research. The reason could be attributed to the methodology of our study and other factors, such as varying time intervals between testing and dialysis and the implementation of efficient measures to decrease the occurrence of hypoalbuminemia.
Patients undergoing chronic HD using venous catheters have an increased risk of infections and adverse outcomes. These complications can be reduced using arteriovenous fistulas (AVF) for chronic HD. Therefore, the use of double-lumen venous catheters should be limited to the conditions where AVF have not been inserted or is immature or in emergency conditions (19). Using tunneled catheters, adhering to aseptic procedures by both patients and dialysis staff, including hand-washing, wearing face masks, applying antibiotic ointment to the catheter's exit site, identifying S. aureus carriers, using antimicrobial lock solutions, and actively monitoring for infection are essential steps in preventing catheter infections (12).
5.1. Limitations of the Study
The main drawback of this study was the lack of evaluation of any therapies that could enhance the albumin level. Additionally, all patients in the study were already effectively managing their nutritional condition and diet. The relationship between the type of organism and the albumin level can be interpreted with a larger sample size. Criteria such as muscle mass are a better indicator of nutritional status.
5.2. Conclusions
In this study, hypoalbuminemia was not found to be an independent risk factor for catheter-related infections. Staphylococcus aureus and S. epidermidis were the most common microorganisms in catheter-related infections in HD patients.