1. Context
Cervical cancer is the fourth most common cancer in women worldwide. In 2018, 569,847 new cases of cervical cancer were diagnosed globally, and 311,365 people died from this disease (1). The World Health Organization (WHO) has predicted that if appropriate interventions for the prevention and early treatment of cervical cancer are not implemented, the death rate due to this disease will increase by 50% by 2040 (2). In Iran, cervical cancer is the second most common cancer of the reproductive system in women (3). According to the results of studies, the prevalence of cervical cancer increased in Iran between 2003 and 2010 (4-6). On the other hand, in most cases, cervical cancer has been reported in advanced stages (7, 8). Various biological, social, economic, and health factors have been proposed as risk factors for cervical cancer. Many of these risk factors are related to sexual activity and exposure to sexually transmitted diseases (9). Human Papillomavirus (HPV) infection is the most important known cause of cervical cancer, and people at risk of HPV should perform regular screening tests to prevent cervical cancer (10). Cervical cancer is considered a preventable cancer due to the possibility of cytological screening of the cervix, the long interval between the transformation of pre-invasive lesions into invasive lesions, and the effective treatment of pre-invasive lesions (11). The main goal of cervical cancer screening is the timely diagnosis of cellular changes and the immediate treatment of the disease. If the screening program is successful, it is expected that complications and deaths caused by this disease will decrease in society (4). The screening program is a low-cost and effective way to reduce cervical cancer, and it is recognized as an effective method to address cervical cancer in many developed countries (9). On the other hand, in 2018, the WHO proposed a strategy to eliminate cervical cancer worldwide. The elimination strategy of this organization is based on the three basic principles of prevention through vaccination, screening, and treatment of precancerous lesions and invasive cervical cancer (2). Researchers have stated that the negative impact of cervical cancer is greater in developing countries, where the mortality rate among women is higher. Therefore, it is necessary to take cost-effective and culturally acceptable measures to reduce these cancers (12). Economic evaluation, by determining, calculating, and comparing the costs and benefits of health and medical interventions, helps health system policymakers to implement health and medical interventions with higher benefits or greater effectiveness. Economic evaluations help increase the efficiency of the health system by prioritizing and allocating optimal resources. By improving access and equity, they contribute to enhancing the effectiveness of the health system (13). In Goldhaber-Fiebert et al.'s study, the cost-effectiveness of using vaccination, HPV2 capacity, and the primary of Pap smear and HPV screening programs were compared in different age groups. The results showed that, in women who were not vaccinated, cytology should begin with HPV testing at the age of 30. Compared to starting it at 21 years old, it has a higher cost-effectiveness [quality-adjusted life years (QALY) /78,000]. For girls who have been vaccinated, starting cytology with HPV testing at the age of 35 years has a higher cost-effectiveness (QALY/41,000) than starting it at 25 years old (14). The results of the Armstrong study showed that HPV vaccination is highly effective and potentially cost-effective when administered to women without exposure to the HPV virus (15). Also, in the study by Sharifa and Aljunid in Malaysia, the use of the quadrivalent HPV vaccine was reported to be more cost-effective than the bivalent HPV vaccine and Pap smear test (16). While the results of the Techakehakij and Feldman study showed that HPV vaccination is affordable in only 46 countries with high GDP per capita (17). Since cervical cancer is a preventable cancer (11), but as the results of studies are contradictory regarding the cost-effectiveness of this cancer prevention method, there is disagreement about the cost of the most effective prevention method (14-17). More cost-effectiveness studies are needed in developing and low-income countries to inform policy decisions (17). The researcher decided to conduct a narrative review with the aim of comparing the cost-effectiveness of Pap smear, HPV test, and HPV vaccination in cervical cancer prevention.
2. Evidence Acquisition
In order to access the available studies in the field of the subject under review, a search was conducted between the years 1990 and 2025 to review articles published in recent years in the SID, Google Scholar, PubMed, Cochrane, Scopus, and Web of Science electronic databases. All published articles between 1990 and 2025 were investigated. To locate all relevant Persian and English articles, the keywords HPV test, Pap smear, HPV vaccine, and cervical cancer were used. The search strategy involved formulating clinical questions based on the PICO model, which included (P) population: Women and girls eligible for screening; cervical cancer and HPV vaccination; (I) intervention: Pap smear, HPV test, and HPV vaccination; (C) comparison: Cost-effectiveness comparison of Pap smear, HPV test, and HPV2, 4, and 9 vaccinations; and (O) outcome: Cost-effectiveness. The search for these keywords was restricted to the title and abstract of the articles, and studies with ecological, cross-sectional, case-control, prospective cohort, and meta-analysis designs were selected. To ensure a comprehensive search, the reference lists of all related articles were also reviewed.
Initially, a list of titles and summaries of articles was prepared for studies conducted between 1990 and 2025. The main inclusion criteria of the studies in the present review included articles published between 1990 and 2025 in which the cost-effectiveness of three methods — Pap smear, HPV test, and HPV vaccination — was compared. The inclusion criteria also encompassed the use of reliable sources, valid methods for data collection and analysis, and the specificity of the objectives, methodology, and results. Exclusion criteria included low-quality articles, inappropriate or out-of-scope content, papers presented at conferences, letters to the editor, and insufficient data in the articles.
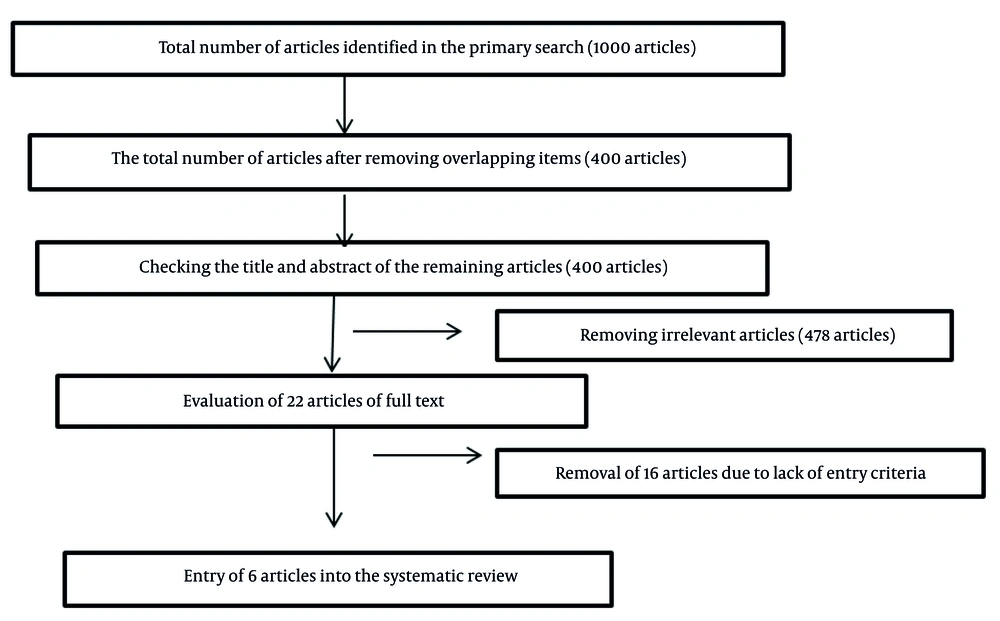
In the initial search, 1000 articles were found. After removing duplicates and unrelated studies, 400 articles were examined. After reviewing the titles and abstracts of these 400 articles, 22 articles were examined in full text. Of these, after applying the inclusion and exclusion criteria, 6 articles were selected for this review (Figure 1). In the next step, after determining the selected articles, the researchers qualitatively evaluated the articles using the Strobe checklist. The purpose of presenting this form is to provide recommendations for making the design, implementation methods, and findings of observational studies as clear as possible. In other words, the purpose of the Strobe statement is to report studies in the best possible manner. The Strobe checklist consists of 6 general sections titled: Title and abstract, introduction, methods, results, discussion, and other information. Some of these sections are further divided into subcategories, and in total, this statement contains 22 items (18). After reviewing the full text of seemingly related articles using the Strobe checklist, the articles that had the highest scores and were most relevant to the purpose of the present study were selected and analyzed. All the final articles included in the study process were entered into a pre-prepared checklist containing the names of the authors, the year of the study, the location of the study, the study method, the tools used, and the results. In the present study, the findings were investigated from two perspectives: (1) Cost-effectiveness comparison of Pap smear and HPV test in cervical cancer screening, and (2) cost-effectiveness comparison of Pap smear, HPV test, and HPV vaccination in cervical cancer screening.
3. Results
In the initial search, 1000 articles were found. After removing duplicates and unrelated studies, 400 articles were reviewed. After reviewing the titles and abstracts, 22 articles were reviewed in full text. Of these, after checking the inclusion criteria, 6 articles were finally selected for this review (Figure 1).
The results were analyzed in terms of comparing the cost-effectiveness of the Pap smear and HPV test in cervical cancer screening, as well as comparing the cost-effectiveness of the Pap smear, HPV test, and HPV vaccination in cervical cancer screening (Table 1).
| Author/Year Reference | Location Study | Method | Tools | Results |
|---|---|---|---|---|
| Chow et al. (2010) (19) | Taiwan | Comparison of Pap smear alone and HPV DNA test with Pap smear, with three annual screening intervals, every 3 years, and every 5 years | Using clinical results and cost-effectiveness in terms of QALY | The cost of Pap smear alone was 660,000/QALY every 3 years and 889,000/QALY every 5 years, and Pap smear with HPV every 3 years was 1,323,000/QALY and every 5 years was 1,358,000/QALY. The cost of annual Pap smear was lower compared to HPV every 3 years and 5 years. |
| Van Rosmalen et al. (2012) (20) | Netherlands | Comparison of Pap smear alone and HPV DNA test with Pap smear | Using cost-effectiveness in QALY | Primary HPV testing with a Pap smear is the most cost-effective strategy, and its cost-effectiveness was €20,000 per QALY. |
| Kulasingam et al. (2009) (21) | Canada | Comparison of Pap smear alone and HPV DNA test with Pap smear. | Using cost-effectiveness in QALY | Primary HPV testing with a Pap smear repeated every 5 years was the most cost-effective strategy, and its cost-effectiveness was $20,000 per QALY. |
| Mo et al. (2017) (22) | China | Comparison of the use of HPV vaccination 2, 4, and 9 capacity in Pap smear screening program alone and HPV DNA test together with Pap smear | Using clinical results and cost-effectiveness in terms of QALY | Screening with HPV DNA test along with pap smear plus 9-valent HPV vaccination (Gardasil9) had a higher cost-effectiveness/QALY of 24.867 and showed the best effect in prevention, reducing the incidence of cervical cancer by 34.39% and reducing cancer deaths by 35.95%. There was a 25.82% reduction in HPV infection and a 20.80% reduction in genital warts, but the use of the HPV2 vaccine was not cost-effective. |
| Chen et al. (2010) (23) | Taiwan | Cost-effectiveness comparison of Pap smear, HPV test, and vaccination | Cost-effectiveness in QALY | Cost-effectiveness was highest for annual Pap smears ($31,698), followed by HPV DNA testing with Pap smears every 3 years ($36,627), and then the vaccination program with triennial Pap smear screening ($44,688). |
| Goldhaber-Fiebert et al. (2008) (14) | America | Comparison of the cost-effectiveness of starting a Pap smear screening program and HPV vaccination use, HPV2 capacity | Cost-effectiveness questionnaire | For unvaccinated women, starting cytology with HPV testing at age 30 compared to starting at age 21 has a higher cost-effectiveness (QALY/78,000), and for vaccinated girls, starting cytology with HPV testing at age 35 compared to starting at age 25 has a higher cost-effectiveness (QALY/41,000). |
Abbreviations: QALY, quality-adjusted life year; HPV, human papillomavirus.
3.1. Cost-Effectiveness Comparison of Pap Smear and Human Papillomavirus Test in Cervical Cancer Screening
There is disagreement about comparing the cost-effectiveness of annual Pap smears with HPV testing. In the study by Chow et al., the effective cost of a yearly Pap smear was lower compared to HPV testing every 3 years and 5 years (19). However, in the study by Chen et al., the effective cost of an annual Pap smear was higher compared to HPV testing every 3 years (23). In other studies, the use of Pap smear with HPV testing (co-testing) was found to be the most cost-effective strategy in cervical cancer screening (20, 21).
3.2. Comparing the Cost-Effectiveness of Pap Smear, Human Papillomavirus Test, and Human Papillomavirus Vaccination (2-Cervarix, 4-Gardasil, and 9 Capacity) in Cervical Cancer Prevention
The results of reviews showed that there is disagreement about the cost-effectiveness of HPV vaccination (19, 22). In the study by Mo et al., the cost-effectiveness of screening with an HPV DNA test along with a Pap smear in addition to HPV vaccination with 9 capacity was higher, but the use of HPV vaccination with 2 capacity had low cost-effectiveness and was not affordable (22). However, in the study by Goldhaber-Fiebert et al. (14), the use of HPV2 vaccination was cost-effective, reduced the frequency of sampling, and increased the age of screening in vaccinated women. The current review study was conducted with the aim of comparing the cost-effectiveness of Pap smears, HPV tests, and HPV vaccination in cervical cancer screening. The results showed that in most studies, the use of co-testing has higher cost-effectiveness than the Pap smear test alone (19, 20, 23). However, in the study by Jin et al., the primary HPV test detected more cases of CIN3+ compared to the primary Pap smear and co-testing, and had a higher specificity than these two tests. The primary HPV test introduced a cost-effective alternative to concurrent testing (24), which is not consistent with our study. Meanwhile, the results of the study by Felix et al. showed that co-testing had a higher cost-effectiveness compared to the primary HPV test and had the potential to provide higher clinical and economic results compared to the primary HPV test (25). Miller et al. also reported that co-testing had more QALYs compared to primary HPV and reduced the mortality rate from cervical cancer by 45 - 48% (26). Choi et al. reported that co-testing and liquid-based cytology test alone had slightly higher positive predictive values for CIN 2+ than primary HPV screening alone (27). These findings were consistent with those of the present study.
Cervical cancer is considered a preventable cancer due to the possibility of cytological screening of the cervix, the long interval between the transformation of pre-invasive lesions into invasive lesions, and the effective treatment of pre-invasive lesions (11). The main goal of cervical cancer screening is the timely diagnosis of cellular changes and immediate treatment of the disease. If the screening program is successful, it is expected that complications and deaths caused by this disease will decrease in society (28). Human papillomavirus infection is the most important known cause of cervical cancer, and individuals at risk of HPV should perform regular screening tests to prevent cervical cancer (10). Some researchers believe that the increase in the probability of this cancer in Iranian women in recent years may be due to the rise in HPV infection. Khodakarmi et al., in their study on 825 women aged 18 - 59 years in Tehran, reported an HPV prevalence of 7.8% (29). The results of the study by Jamdar et al. also showed that the prevalence of HPV infection in 2435 women referred to medical centers in Tehran was 10.3% (30). On the other hand, the results of a study conducted in Isfahan city by Allameh et al. indicated that among 80 women aged 18 to 60 who referred to specialized clinics for women and childbirth in Isfahan city, 25.55% tested positive for HPV, and in 15.21% type 16 and 13.04% type 18 were reported (31). Given the prevalence of the HPV virus and its known role as the cause of cervical cancer, people at risk for HPV should perform regular screening tests to prevent cervical cancer (10). Therefore, cost-effective and culturally acceptable measures should be taken to reduce this cancer (12). Additionally, the use of co-testing can be identified as one of the most effective methods of cervical cancer prevention (20, 21, 26).
The results of our study showed that there are differences of opinion regarding HPV vaccination, particularly Gardasil 2, but HPV vaccination with 9-valent capacity is considered a highly cost-effective interventional method for preventing cervical cancer (19, 22). In the study by Kim et al., the effectiveness of HPV vaccination for women over 30 years of age who are screened is also low. The cost-effectiveness of HPV vaccination is lower than co-testing, and co-testing is well accepted compared to other interventions in the United States (32). These findings were consistent with those of the present study. The HPV vaccine has been available in most high-income countries since 2006 (33). According to studies, in developed areas, 33.6% of women aged 10 - 20 years have used HPV vaccination, but in developing countries, only 2.7% of women have used the HPV vaccine to prevent cervical cancer (34). In the study by Techakehakij and Feldman, the use of the HPV vaccine was cost-effective only in countries with high GDP per capita, and it was not cost-effective in developing countries (17). Although HPV vaccination is known as one of the most effective methods worldwide to prevent cervical cancer, it is not part of Iran's vaccination program due to reasons such as insufficient knowledge about the vaccine, people's concerns about the safety of the vaccine, and the high cost of the vaccine. In Iran, this method is not used to prevent cervical cancer (35-37). Since most vaccines provide protection only against high-risk HPV viruses, vaccinated women should also perform Pap smears regularly (11). On the other hand, the results of studies have shown that vaccination alone will have less effect on preventing cancer-related deaths. Given that vaccines only provide protection against high-risk HPV viruses, vaccinated women should also perform a Pap smear regularly (5, 34). The use of vaccination results in a 0.1% decrease in cervical cancer deaths, while twice screening by Pap smear testing during a lifetime reduces cervical cancer deaths by 20 - 30% (2).
4. Conclusions
The results of the review of studies showed that in most cases, the use of co-testing has a higher cost-effectiveness than the Pap smear test alone. Therefore, the use of this method is recommended as the most cost-effective approach for the prevention of cervical cancer. However, there is a difference of opinion regarding the cost-effectiveness of HPV vaccination, and more studies are needed to evaluate the cost-effectiveness of vaccination against HPV.