1. Background
Salmonella is a type of millipede and gram-negative bacteria that cannot ferment lactose and sucrose. The pathogenic mechanism of Salmonella in humans includes Salmonella typhi and Salmonella paratyphi, which are adapted to the human host (1, 2). Salmonella is one of the major public health concerns of the poultry industry. Poultry, especially chickens, are known as the main carriers of Salmonella to humans (1-4). Salmonellosis disease in humans is transmitted by eating undercooked chicken meat or other poultry products contaminated with Salmonella due to cross-contamination with raw chicken meat. Children, the elderly, and those with immunodeficiency are at risk for severe diarrhea, which may require hospitalization and drug therapy. This bacterium can also enter the bloodstream from the intestine and cause septicemia and death (5). Meanwhile, in the last thirty years, antibiotic resistance has been seen in Salmonella, which has become a growing global concern regarding public health (6, 7).
As chicken meat consumption has increased per capita, contamination with bacteria or chemicals increases the risk of poisoning in consumers (8-10). Meanwhile, processing places, especially poultry slaughterhouses, play an essential role in spreading and transmitting pollution. In addition, the carcasses obtained in the slaughterhouses are contaminated by contaminating the environment, personnel, equipment, and secondarily. Finally, the infection is transferred to humans by consuming this product, and depending on the number of bacteria, a chronic or acute form of the disease occurs (11-13). The joint report of the Food and Agriculture Organization of the United Nations (FAO), based on numerous studies, has shown that the type of serotype, the kind of food, and the conditions of the host have a decisive role in the pathogenicity of this bacterium (14).
Evaluating broiler chicken carcasses in terms of Salmonella contamination can help effectively control the contamination of these meat products. Although rapid detection and differentiation of Salmonella serotypes remains a challenge for the food industry, recent advances in molecular methods have made Salmonella detection more accurate and convenient (15-17). Access to correct and precise information related to the spread of Salmonella in chicken farms can be effective in monitoring the sources and routes of microbial contamination by health officials. Thus, control and prevention programs can be developed and implemented to reduce or eliminate contamination by this bacteria (18-20).
2. Objectives
Considering the importance of Salmonella control in chicken slaughterhouses, the main objective of the present study was to evaluate Salmonella contamination in slaughtered broiler chickens (SBCs) in slaughterhouses in Golestan province, Iran.
3. Methods
3.1. Sampling
Golestan province is located in northern Iran and reaches Turkmenistan from the north, Semnan Province from the south, North Khorasan province from the east, and Mazandaran province and the Caspian Sea from the west (Figure 1). The area and population of this province are 20438 km2 and 1868819 people, respectively. To conduct this study, frozen chicken samples were taken for analysis during January, February, and March in 2024. Accordingly, 12 chicken slaughterhouses in Golestan province were selected by census. Every month, samples were taken from each slaughterhouse three times (every ten days), and five samples from five different cartons were randomly selected. Based on this, a total of 540 frozen chicken samples (3 × 3 × 5 × 12 = 540) were collected to evaluate Salmonella contamination and the total number of microorganisms (TNM) throughout Golestan province (Table 1).
| Chicken Slaughterhouse | Number of Collected Samples | Total | ||
|---|---|---|---|---|
| Sampling Months | ||||
| January | February | March | ||
| A-CS | 15 | 15 | 15 | 45 |
| B-CS | 15 | 15 | 15 | 45 |
| C-CS | 15 | 15 | 15 | 45 |
| D-CS | 15 | 15 | 15 | 45 |
| E-CS | 15 | 15 | 15 | 45 |
| F-CS | 15 | 15 | 15 | 45 |
| G-CS | 15 | 15 | 15 | 45 |
| H-CS | 15 | 15 | 15 | 45 |
| I-CS | 15 | 15 | 15 | 45 |
| J-CS | 15 | 15 | 15 | 45 |
| K-CS | 15 | 15 | 15 | 45 |
| L-CS | 15 | 15 | 15 | 45 |
| Total selected samples | 540 | |||
Abbreviation: CS, chicken slaughterhouse.
3.2. Analysis of Samples to Identify Salmonella and Total Count of Microorganisms
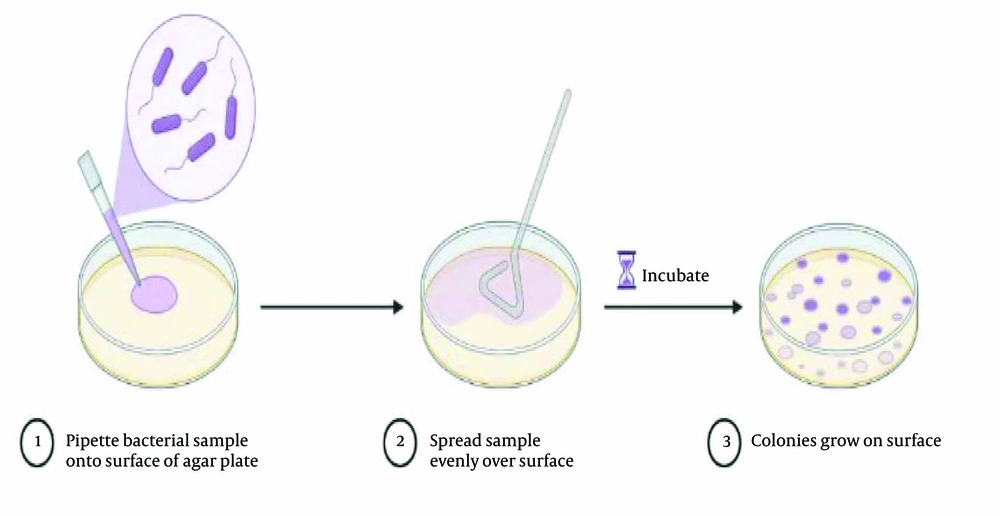
The Institute of Standards and Industrial Research of Iran (ISIRI) method (No. 1810-1) was used to identify Salmonella in the selected samples (21). According to this method, 25 g of each sample was weighed and mixed with 225 mL of lactose broth on the first day of sample analysis. Finally, the obtained mixture was placed in a sterile container for 24 hours in a water bath with a temperature of 37°C. On the second day, 1 cm3 of the culture of the first day was added to the tube containing 10 mL of tetrathionate culture medium, and then it was placed in a water bath at 37°C for 24h. In addition, the same operation was performed on a cysteine selenite culture medium, which was put in a water bath at a temperature of 37°C. On the third day, the culture tubes of the second day were taken out of the greenhouse, and a culture ring was removed from the culture tubes of selenite and tetrathionate medium separately with Anse Platin. Then, each was placed in two Petri dishes, one containing Salmonella-Shigella agar (SS agar) and the other containing Brilliant green agar. Finally, the prepared plates were placed in a water bath at 37°C for 24h. On the fourth day, the plates of the third day were examined for the growth of Salmonella bacteria in the culture medium. For the total count of microorganisms, the surface counting method (Figure 2) was performed according to ISIRI (No.5272-2) (22).
3.3. Statistical Analysis
The data were analyzed using SPSS 22 software. The Kolmogorov-Smirnov test determined the normality of the data distribution. A two-way ANOVA test compared the results between different groups, and Duncan's post hoc test compared two groups (pairwise comparisons). For all three statistical tests, the level of statistical significance was considered equal to α = 0.05.
4. Results
In the current study, 540 frozen chicken samples were analyzed to detect Salmonella, and the results showed that Salmonella was not detected in any of the samples. In addition, the Log value of the TNM was 2.81 ± 0.16 cfu/g on average, and this value is presented in Tables 2 and 3 based on months and the evaluated slaughterhouses, respectively.
Table 4 presents the effect of the slaughterhouse, sampling time, and their interaction effect on TNM count. There was a significant interaction effect between slaughterhouses and time, which means that different levels of sampling time have different effects on TNM in chicken meat, and the reverse is also true. In addition, the results showed that the impact of slaughterhouse type on TNM in chicken meat is significant (P < 0.001).
| Study Months | Sample Size | Log of the TNM, cfu/g |
|---|---|---|
| January | 180 | 2.39 ± 0.17 |
| February | 180 | 2.39 ± 0.17 |
| March | 180 | 2.37 ± 0.14 |
| Total | 540 | 2.81 ± 0.16 |
a Values are presented as mean ± SD.
| Chicken Slaughterhouse | Sample Size | Log of the TNM, cfu/g |
|---|---|---|
| A-CS | 45 | 2.54 ± 0.07 |
| B-CS | 45 | 2.53 ± 0.10 |
| C-CS | 45 | 2.43 ± 0.14 |
| D-CS | 45 | 2.42 ± 0.09 |
| E-CS | 45 | 2.41 ± 0.10 |
| F-CS | 45 | 2.39 ± 0.10 |
| G-CS | 45 | 2.39 ± 0.18 |
| H-CS | 45 | 2.36 ± 0.16 |
| I-CS | 45 | 2.35 ± 0.11 |
| J-CS | 45 | 2.32 ± 0.15 |
| K-CS | 45 | 2.29 ± 0.15 |
| L-CS | 45 | 2.21 ± 0.17 |
| Total | 540 | 2.81 ± 0.16 |
a Values are presented as mean ± SD.
b Log: logarithm, cfu/g: colony forming per gram; CS, chicken slaughterhouse.
| Variables | df | Mean Square | F | P |
|---|---|---|---|---|
| Slaughterhouse | 11 | 0.3791 | 27.151 | < 0.001 |
| Sampling time | 2 | 0.0613 | 4.393 | 0.013 |
| Slaughterhouse × sampling time | 22 | 0.0992 | 7.104 | < 0.001 |
5. Discussion
The results showed that Salmonella was not detected in any of the samples of SBCs, indicating the outstanding microbial quality of SBCs in terms of Salmonella. In addition, the average TNM Log value was 2.81 ± 0.16, less than the permissible limit (log 10 CFU/g 6 5 to) in all slaughterhouses and sampling months (23). In addition, both investigated variables (slaughterhouse and sampling time) and their interaction on TNM were significant, indicating that different spatial and temporal conditions can effectively affect the level of SBC contamination with Salmonella. The absence of Salmonella contamination in SBC samples reflects the complete poultry inspection and the observance of hygiene principles in the slaughterhouses of Golestan province. Furthermore, this lack of contamination can be attributed to proper management in chicken farms and the use of particular antibiotics in chicken production in Golestan province. Thus, a separate study is necessary to determine the correct reason for this issue.
Unlike the absence of Salmonella contamination in SBC samples evaluated in the present study, in other similar studies conducted at different times and places in Iran, different prevalences of Salmonella contamination in SBC samples have been reported. Miahi et al. conducted a study on the contamination of poultry products to Salmonella in poultry farms in Ahvaz, Iran. The results of the mentioned study showed that out of 31 chicken farms evaluated, 18 chicken farms (58.1%) and 71 out of 930 samples (7.63%) were infected with Salmonella (24). The study of Doulatyabi et al. reported that out of a percentage of 188 broiler samples, 1.8% were infected with Salmonella (25). The mentioned percentage of Salmonella contamination in broiler samples in other parts of Iran, including Tehran, the whole country of Iran, Amol, and Ghaemshahr, were obtained as 35.29%, 10.83%, 27.43%, and 13.15%, respectively (26-29).
In many cases, Salmonella bacteria remain in the internal organs as a hidden infection without showing clinical symptoms and bacterial excretion, making it difficult to search for it in feces (30). In other words, Salmonella's isolation rate from different organs is not the same. The best organs for isolating Salmonella include the cecum, liver, and yolk sac, and with increasing age, bacteria can be isolated only from the liver and cecum (28, 30). Therefore, the isolation of Salmonella from feces and cloacal swabs cannot indicate the actual prevalence of Salmonella in flocks, and the contamination rate of chicken flocks is much higher than the findings of this type of evaluation (31). Suppose it is possible to access the carcass of birds. In that case, it is recommended to use samples of internal organs, including the liver and cecum, along with fecal samples to isolate Salmonella (25).
In some studies, the prevalence of Salmonella in broilers has been reported to be high. For example, the results of Bokaie et al. in 23 provinces of the country, in determining the prevalence of Salmonella infection in broiler farms and also determining the relationship between management factors and Salmonella infection in these farms, showed that out of 139 sampled farms, 11 farms (7.9%) were infected with Salmonella bacteria and Tehran and Fars provinces had the highest number of infected cases. Based on the results, the age of chickens and the number of poultry houses are essential risk factors for Salmonella infection. According to the study's report, the prevalence of Salmonella infection in the country's chicken farms was relatively high (32). Sadeghi et al. reported that out of 1440 samples of SBCs evaluated in the Urmia industrial slaughterhouse, 300 samples (20.83%) were infected with Salmonella bacteria (33). Shafii-Dastgardi et al. reported this contamination level as 11.8% (34).
Unlike the research mentioned above, in other studies conducted in Iran regarding Salmonella contamination in SBC samples, the level of contamination has been reported to be low. In the study of Akbarian et al., equal to 3.84% (27); in the study of Zare Bidaki et al., equal to 2.8% (35) and in the study of Peighambari et al. reported equal to 1.9% (36).
The difference in the results of the studies may be because the studies were conducted in regions with different climatic conditions. Consistent evidence shows gastrointestinal infection with bacterial pathogens such as Salmonella positively correlates with ambient temperature, as warmer temperatures allow for faster proliferation (37). In addition to rising temperatures, poor sanitation can affect the entire food chain, from food production to food consumption. Production, processing, transportation, preparation or storage, and even the kitchen may allow pathogens to multiply and lead to an increase in patients with intestinal infections (38). The storage conditions of poultry carcasses can also play a role in the growth of Salmonella. For example, it has been reported that extensive contact between carcasses may contribute to cross-contamination (39). On the other hand, Ahmed et al. found that freezing chicken carcasses at a temperature of -18℃ eliminates the positive presence of Salmonella in them because the low temperature damages the bacterial cell wall and leads to the death of the pathogen (40).
In addition to the factors mentioned above, different sample sizes in different studies, sampling from different chicken components, sampling from different sections of hatchery factories, different poultry feed factories, etc., can lead to different results. Since chicken meat is widely consumed in Iran, its contamination with pathogenic bacteria such as Salmonella causes food poisoning in people, which has health and economic consequences. It is essential to know its dimensions and details to prevent this disease. Without a detailed assessment of the spread of Salmonella in eggs and poultry meat, the extent of the problem cannot be understood. Therefore, identifying pollution sources can temporarily interrupt society's infection cycle.
5.1. Limitations
One limitation of the present study was the lack of evaluation of different components of broiler carcasses for Salmonella detection. Another limitation was the lack of assessment of broiler samples before their freezing.
5.2. Conclusions
Based on the results, during the three-month research period, the meat of chickens slaughtered in any of the 12 industrial poultry slaughterhouses in Golestan province, Iran, was not contaminated with Salmonella because Salmonella bacteria were not isolated and observed from any of the 540 examined samples. In addition, in counting the TNM, the average logarithm was 2.8138, which was lower than the limit of TNM. The results showed that the poultry slaughtered in the slaughterhouses of Golestan province are in good condition regarding the presence of microorganisms.