1. Background
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder, significantly impacting millions worldwide (1). It is characterized by recurrent episodes of partial or complete upper airway obstruction during sleep, leading to disrupted breathing, oxygen desaturation, and fragmented sleep patterns (2). Evidence estimates that approximately 936 million adults aged 30 - 69 suffer from mild to severe OSA, while 425 million experience moderate to severe forms (3). The OSA has been linked to increased risks of cardiovascular diseases such as hypertension, myocardial infarction, and stroke, as well as metabolic disorders like diabetes mellitus (4, 5). It also impairs cognitive functions and productivity, with patients experiencing difficulties concentrating and learning new tasks, further reducing their quality of life and workplace performance. Research has shown that individuals with OSA are nearly twice as likely to be involved in occupational accidents compared to their counterparts (6, 7). Economically, OSA imposes significant healthcare costs, including diagnostic evaluations such as polysomnography, treatments like continuous positive airway pressure (CPAP) therapy, and management of associated conditions; moreover, untreated OSA contributes to indirect costs through reduced productivity and absenteeism (4). Policymakers must address these implications to allocate resources effectively and develop cost-efficient strategies (8). Research conducted in western Iran highlights the public health concern, as over 27% of individuals are at high risk of developing OSA, while many remain undiagnosed (9).
2. Objectives
This study aimed to estimate the economic burden of OSA in Kermanshah, where over a quarter of adults are at high risk (9). Understanding OSA’s prevalence and impact is essential for targeted interventions to reduce its health, social, and economic consequences, particularly in regions like Iran with limited data on its financial implications.
3. Methods
3.1. Sample Recruitment
This cross-sectional study recruited 179 patients with OSA, diagnosed using Polysomnography (PSG), as recommended by Kapur et al. (10). All diagnoses were confirmed by a neurosurgeon at the Sleep Research Center of Kermanshah University of Medical Sciences (KUMS) in western Iran. Kermanshah, with a population of 946,651 (2016 census), served as the study site. A cost of illness (COI) prevalence-based approach was applied to calculate the economic burden of OSA, covering direct healthcare, direct non-healthcare, and indirect costs (11). This method estimates costs over one year, offering policymakers insights for resource allocation and intervention development.
Simple random sampling was employed to select patients referred to the Sleep Research Center at KUMS. Using their medical record numbers, randomized lists were created, and data collection was conducted via telephone interviews between January and September 2020 by a trained public health expert. The PSG diagnoses were carried out one year prior to data collection. Participants unwilling to answer questions were excluded. Annual costs were calculated for eligible patients confirmed with OSA via PSG.
3.2. Instruments
A two-part questionnaire was the primary data collection tool, refined through iterative expert feedback from specialists in sleep disorders, general practice, psychiatry, public health, health policy, and health economics. This process ensured the validity, reliability, and alignment of the questionnaire with the study goals.
3.2.1. Background Information
The questionnaire included questions to capture demographic and socioeconomic details, such as diagnosis duration, age, sex, marital status, education level, weight, height, health insurance, supplementary health insurance, place of residence, and monthly income.
3.2.2. Obstructive Sleep Apnea-Related Costs
Costs were calculated in Iranian Rials and converted to US dollars ($1 = 42,000 Iranian Rials). This included direct healthcare costs, direct non-healthcare costs, and indirect costs, adapted from previous disease burden studies (12, 13).
3.2.3. Direct Healthcare Costs
These covered medications, health center visits, complementary therapies (e.g., CPAP usage), surgeries, and diagnostic procedures like PSG and CT scans.
3.2.4. Direct Non-healthcare Costs
These included transportation to clinics, suspension due to visits, and pharmacy trips.
3.2.5. Indirect Costs
These assessed lost workdays, expenses for food and accommodations, family care costs, and incidents like work or drowsiness-related car accidents over the past year.
3.3. Data Analysis
Data analysis utilized Excel and STATA software (version 14.2). Total disease costs were calculated by combining direct and indirect costs, with results presented as mean costs per patient. Univariate analysis was conducted to examine relationships between costs and explanatory variables. The Kruskal–Wallis and Mann–Whitney tests were used to address non-normal distributions. A pie chart illustrated the distribution of OSA-related costs across categories.
4. Results
The mean age of patients was 48.04 years [95% CI: 46.24, 49.84], ranging from 12 to 74 years. The study included 179 patients with OSA, where 65.9% were male and 34.1% female. The majority belonged to the 46 - 60 age group (41.9%) and were married (84.9%). Most participants resided in urban areas (72.1%) and were self-employed (53.6%). Educational levels varied, with 33.5% having less than a diploma, 38.5% holding a diploma, and 28% pursuing higher education. BMI analysis showed that 81% were overweight or obese. Monthly household income spanned three categories: Less than $500 (14%), $500 - 1000 (27.4%), and more than $1000 (30.1%). Diagnoses ranged from less than one year (32.9%) to over three years (12.3%).
Moreover, 14 participants (7.8%) reported a history of car accidents attributed to drowsiness or reduced concentration as a result of OSA. Furthermore, workplace incidents related to drowsiness were reported by 5% of the patients (9 out of 179 patients), while 20.7% (37 out of 179 patients) experienced absenteeism from work due to the same issue. These findings highlight the significant impact of OSA on daily functioning and safety.
The findings indicated that all patients utilized physician specialist visits and underwent PSG testing (100%). However, the use of other resources was limited, with only 8.9% undergoing CT scans, 30.7% using medications, and just 1.1% adopting CPAP therapy. The analysis of descriptive data and costs among 179 patients revealed no statistically significant differences in mean costs across variables, as all P-values exceeded 0.05. For example, the mean cost for females ($185) and males ($181) showed no meaningful disparity. Costs varied slightly by age, education level, BMI, residence area, marital status, income, and time since diagnosis, but none showed statistically significant associations. Patients with academic education ($196) and normal BMI ($197) incurred slightly higher mean costs. These findings highlight uniformity in cost burdens across demographic groups, suggesting that OSA-related expenses are consistent regardless of patient characteristics (Table 1).
| Descriptive Data and Cost Mean | Mean of Cost | SE | P-Value |
|---|---|---|---|
| Gender | 0.161 | ||
| Female | 185 | 58 | |
| Male | 181 | 54 | |
| Age groups | 0.300 | ||
| < 30 | 172 | 68 | |
| 31 - 45 | 186 | 51 | |
| 46 - 60 | 185 | 59 | |
| ≥ 61 | 177 | 50 | |
| Years of education | 0.254 | ||
| Under diploma | 188 | 52 | |
| Diploma | 168 | 39 | |
| Academic | 196 | 72 | |
| Time of diagnosis (y) | 0.453 | ||
| < 1 | 197 | 70 | |
| 1 - 2 | 177 | 44 | |
| 2 - 3 | 168 | 46 | |
| ≥ 3 | 184 | 45 | |
| Marital status | 0.203 | ||
| Single | 197 | 64 | |
| Married | 180 | 53 | |
| Residence area | 0.418 | ||
| Urban | 181 | 48 | |
| Rural | 187 | 72 | |
| BMI categories | 0.079 | ||
| Underweight (< 18.5) | 143 | - | |
| Normal (18.5 - 24.9) | 197 | 77 | |
| Overweight (25 - 29.9) | 177 | 57 | |
| Obese (> 30) | 183 | 42 | |
| Income monthly households ($) | 0.806 | ||
| < 500 | 198 | 60 | |
| 500 - 1000 | 183 | 56 | |
| > 1000 | 180 | 51 |
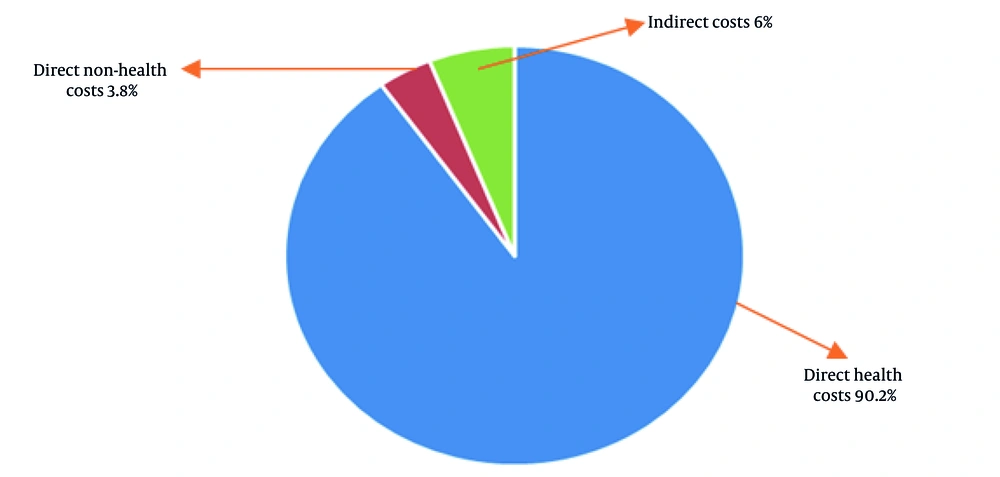
Total annual costs of OSA among patients are shown in Table 2. The total annual costs of OSA among 179 patients amounted to $32,685, with an average cost of $183 per patient. Direct healthcare costs, including treatments and diagnostic procedures, dominated at $29,470 (90.2% of the total), averaging $165 per patient. Direct non-healthcare costs, such as transportation, were much lower at $1,244 ($7 per patient), while indirect costs, accounting for lost productivity and related expenses, totaled $1,971 ($11 per patient). The combined direct costs reached $30,714, underscoring the significant economic burden posed by healthcare-related expenditures.
| Cost Components | Costs Per Patients (Mean ± SD) | Total (n = 179) $USA |
|---|---|---|
| Direct healthcare costs | 165 ± 40 | 29,470 |
| Direct non-health costs | 7 ± 25 | 1,244 |
| Indirect costs | 11 ± 23 | 1,971 |
| Total direct costs | 172 ± 49 | 30,714 |
| Total costs | 183 ± 55 | 32,685 |
Figure 1 depicts the components of the total cost of OSA, including both direct and indirect costs. As shown in Figure 1, direct healthcare costs represented the main part of the costs, accounting for approximately 90.2% of the total expenses.
5. Discussion
This study aimed to estimate the economic burden of OSA in western Iran. The findings revealed a total cost per patient of $182.6, with over 90% attributed to direct medical costs. These expenses include diagnostic procedures such as polysomnography, treatments like CPAP therapy, physician consultations, and medication use. Addressing direct medical costs is crucial, as they represent the immediate financial impact on patients and healthcare systems. Comparative studies, such as Streatfeild et al.’s research in Australia, revealed significant economic burdens from sleep disorders, including OSA, which accounted for $13.1 billion during 2019 - 2020 (14). Differences between studies may arise from varying healthcare tariffs, economic conditions, and healthcare infrastructures.
Given the scope limitations of this study, future nationwide research in Iran is needed to assess OSA’s overall economic impact comprehensively, including direct and indirect costs, for better policymaking and resource allocation. A notable finding was the low CPAP uptake, reported by only 1.1% of participants. The CPAP is recognized as the gold standard treatment for OSA (10), yet barriers such as affordability, perceived lack of need, and dissatisfaction hinder its adoption, as highlighted by Rezaie et al. (15). This low utilization impacts cost estimates by reducing direct medical costs, despite the proven efficacy of CPAP in mitigating disease progression and related complications. Policies to reduce costs, improve insurance coverage, and promote treatment adherence are essential steps toward enhancing OSA management and reducing its long-term burden.
Safety implications were apparent, as 7.8% of patients reported drowsiness-related car accidents. Ellen et al. highlighted the financial impact of OSA-related crashes in the U.S., with over 800,000 drivers involved annually, causing $15.9 billion in damages (16). Hoffman et al. demonstrated the cost-saving benefits of CPAP, reducing healthcare expenses and workplace absenteeism among drivers with OSA (17). However, our study did not calculate accident-related costs, which warrants exploration in future Iranian studies. Additionally, 5% of patients experienced workplace accidents, and 20.7% reported absenteeism due to drowsiness, further underscoring OSA’s impact on productivity. Studies in the U.S. estimated the annual cost of undiagnosed OSA at $149.6 billion, with substantial contributions from lost productivity, workplace accidents, and associated medical conditions (18). Investigating these costs within Iran’s context can provide valuable insights for policymakers.
The high prevalence of OSA risk in Kermanshah highlights the need for efficient diagnostic methods. While polysomnography is the definitive diagnostic tool, its high cost suggests alternative approaches, such as limited-channel sleep tests, could reduce financial strain (19). Policymakers should prioritize accessible and cost-effective diagnostic options to mitigate OSA’s burden on the healthcare system in western Iran.
5.1. Limitations
This study provides the first assessment of OSA’s economic burden in Iran but has limitations. It was limited to a single center in Kermanshah, reducing generalizability. Participants were only those with PSG-confirmed diagnoses, potentially underestimating costs given OSA's high prevalence. Relying on retrospective telephone interviews could lead to recall bias. Significant cost contributors, including expenses arising from accidents and absences due to sleepiness, were not analyzed. Comorbidities like COPD and cardiovascular issues, significant cost drivers, were not examined. Lastly, data from 2020 may underestimate costs due to rising healthcare expenses.
5.2. Conclusions
In conclusion, this study highlights the substantial economic burden of OSA in western Iran, with direct medical costs constituting over 90% of expenses. Addressing barriers to CPAP therapy uptake, such as affordability and awareness, is critical for reducing long-term costs and improving treatment adherence. Policymakers should prioritize cost-effective diagnostic methods, such as limited-channel sleep tests, to mitigate financial strain on healthcare systems. Future research is needed to evaluate OSA’s nationwide economic impact, including costs from accidents, absenteeism, and comorbidities. These insights can guide targeted interventions and health policies to alleviate OSA's burden comprehensively.