1. Background
Cancer is the uncontrolled, rapid, and abnormal proliferation of cells. In other words, cancer cells lose control of the cell cycle and continue to proliferate continuously, regardless of cellular signals and growth factors. In cancer cells, the expression of cellular proteins is altered, and some of them that should not be expressed are expressed, or the expression of some other genes is stopped (1). Colorectal cancer (CRC) or colon cancer occurs when the cells lining the colon or rectum become abnormal and grow out of control. Since symptoms often do not appear until the cancer is advanced, it is important to have regular screening for CRC (2, 3). The CRC is the third most common cancer in men and the second most common cancer in women. The highest rates of this cancer are reported in Australia, New Zealand, Europe and North America, while the lowest rates are found in Africa and south-central Asia (4-6).
Factors contributing to the development of CRC include both uncontrollable risk factors (including age and genetic factors) and controllable risk factors (including a significant number of environmental risk factors such as smoking and alcohol consumption, lifestyle, exercise and physical activity) that play an important role in the development of CRC (5-8). This cancer is a heterogeneous disease and has at least three main forms including hereditary, sporadic and colitis-associated CRC. Recent evidence has shown that the etiology of CRC can be related to chronic inflammation, gene mutations and epigenetic changes. However, the cause of CRC is still not clearly understood due to the complexity of the tumor (2, 3, 9). In the past, it was thought that CRC arises from the accumulation of genetic and epigenetic changes that cause the transformation of normal colonic epithelium into tumor cells. However, recent evidence has shown that four main steps are involved in the process of this type of cancer, including (1) Genetic and epigenetic changes in the structure of the colorectal epithelium that cause the growth of clonal proliferation of abnormal cells; (2) uncontrolled development of cancer at the molecular and morphological levels; (3) loss of genomic stability; and (4) emergence of clinical evidence of cancer (10).
In addition to the recent evidence, stromal cells also play an important role in colorectal tumorigenesis. It has been observed that immune cells such as lymphocytes and macrophages often invade the tumor microenvironment. Such invasion may be of importance in understanding colorectal carcinogenesis. One of the most important processes associated with this event is the activation of the Cyclooxygenase-2 (COX-2) signaling pathway, which is likely to play an important role in tumor pathogenesis (11-14).
Curcumin is a natural yellow phenolic compound obtained from the root of the turmeric plant and has strong anti-inflammatory properties. Other functions of curcumin include reducing angiogenesis and metastasis, inducing apoptosis in cancer cells, and interfering with the death of these cells (15-17). Structurally, chitosan is a linear chain of random N-acetyl, D-glucosamine units linked by (1,4-β) glycosidic bonds. Chitosan contains amine groups at the C2 carbon after deacetylation in the chitin structure. In fact, when the degree of deacetylation in chitin is more than 60 to 70 percent, it is called chitosan. Studies have shown that chitosan strengthens the immune system in plants and animals, reduces cholesterol, and heals wounds. Chitosan is used for drug and gene delivery to target cells due to its positive charge and ability to bind to negatively charged surfaces, as well as its ease of surface modification. Given these characteristics, chitosan is a suitable candidate for the fabrication of drug delivery nanosystems (18).
2. Objectives
As mentioned above, the COX-2 gene is known to be one of the genes involved in inflammatory and carcinogenic processes, and regulating its expression can be effective in controlling cancer progression. The present study mainly aimed to evaluate the effect of chitosan/curcumin nanoparticles (Ch/Cr-NPS) on the expression of COX-2 genes in CRC of Balb/c mice.
3. Methods
In this experimental study, 40 female Balb/c mice, 35 days old and weighing approximately 16 - 20 g, were used. The mice were purchased from the Pasteur Institute in Amol and transferred to the laboratory animal facility at Islamic Azad University of Babol. The animals were acclimatized to the animal environment for two weeks, and then had free access to sufficient food and water. The temperature of the animal room was set at 22 ± 3°C and the lighting conditions were set at 12 hours of darkness and 12 hours of light (19). The mice were divided into four groups including healthy mice, cancerous mice, cancerous mice treated with Ch/Cr-NPS, and cancerous mice treated with cisplatin.
Cancerous tumors in the cancerous mice group were created by CT26 (murine colorectal carcinoma cell line) (20). CT26 cells were provided in the required amount to be injected into the control mice group, free from any contamination. After injection of drug treatment at a specific dose and within a specific time interval, the expression of COX-2 and reference genes was examined by real-time PCR (21). For better interpretation of the findings of COX-2 gene expression, the GAPDH gene was considered as a reference gene. The treatment of the selected groups of mice was performed by intraperitoneal injection and therapeutic injections were made in an amount of 100 μL per day for 14 days. In addition, no substance was injected into the untreated group of mice. The efficacy of the treatment was examined by daily measurement of tumor diameters in three directions (longitudinal, transverse and height) using a digital caliper with an accuracy of 0.01 mm, and then the tumor volume was measured again. Total RNA extraction from cancer cells was performed using the RNX PLUS kit instructions (20).
In the present study, a stock solution of chitosan and curcumin was used to prepare Ch/Cr-NPS. After preparing a clear solution of chitosan and curcumin, a little tripolyphosphate salt was added to it and 45 minutes were given to charge the solution containing chitosan nanoparticles and to become uniform with curcumin. To remove large impurities, the solution was centrifuged in the dark for 15 minutes at room temperature at 4000 rpm, and the supernatant was stored in the dark for concentration and use in the next step (22, 23).
To evaluate the characteristics and confirm the correct and appropriate synthesis of Ch/Cr-NPS, various techniques including Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and dynamic light scattering (DLS) were used (24), and Excel-Ver.2016 and SPSS-Ver.22 software were used to describe and analyze the resulting data. Excel was used to initially record the raw data, draw descriptive and comparative graphs. Then, SPSS software was used to analyze and compare the results obtained from different study groups. For this purpose, the one-sample Kolmogorov-Smirnov test was first used to assess the normality of the data distribution. To compare the findings obtained from different study groups, one-way ANOVA was used at a significance level (α = 0.05).
4. Results
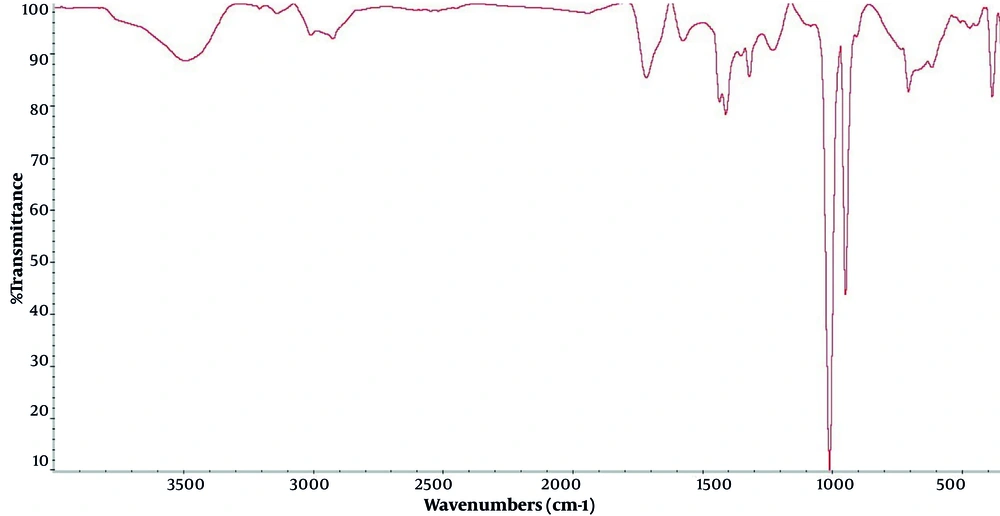
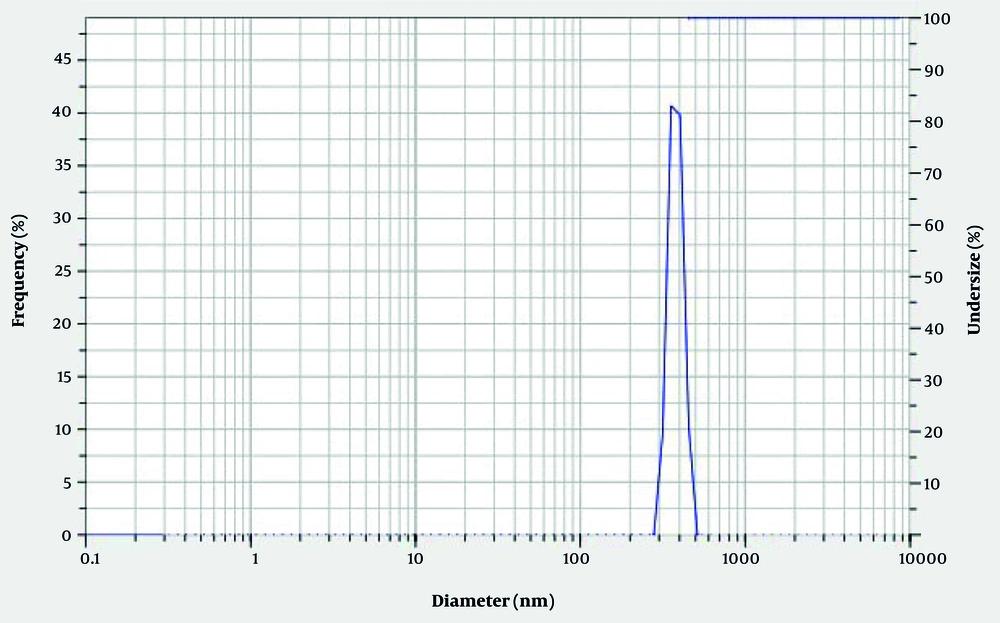
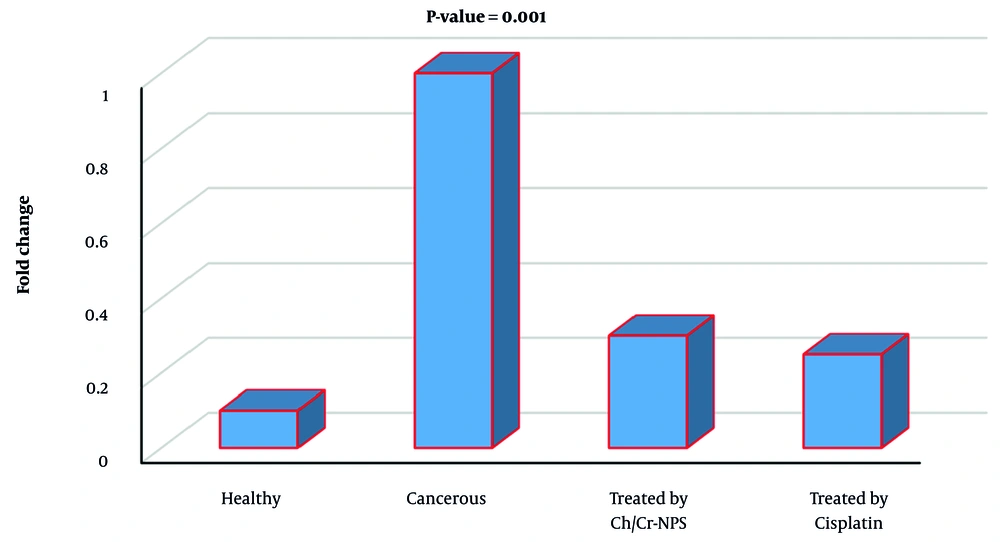
Based on the findings, the analysis of FTIR data of synthesized Ch/Cr-NPS showed that nanocurcumin loaded with chitosan was confirmed by FTIR spectra. In Figure 1, the peaks visible at 1457 cm and 1516 cm were related to aromatic C=C and phenolic C-O groups, respectively, which are only visible in the presence of curcumin in the structure of the compound. In addition, the presence of the band at 1411cm also indicates the presence of chitosan nanoparticles in the synthesized compound. The distribution and average size of the particles in the chitosan-curcumin nanocomposite were determined using DLS. The size of the synthesized nanoparticles was in the range of 100 nm, which was a suitable size for drug delivery (Figure 2). In addition, the results of SEM also confirm the aforementioned findings (Figure 3). Changes in COX-2 gene expression in different study groups can be seen in figure 4, which indicates a significant difference between the group treated with Ch/Cr-NPS compared to other groups.
5. Discussion
The results of the present study showed that COX-2 expression was significantly higher in CRC tissues compared to healthy colon tissues, which is consistent with the study of Negi et al. (25). Meanwhile, the findings of other studies including Soumaoro et al. (14), Yamauchi et al. (26), and Xiong et al. (27) showed that the level of COX-2 gene expression in cancer tissues was almost the same as in healthy tissues.
The exact mechanism of COX-2 overexpression at the molecular level is still unknown. However, several studies have reported that cells overexpressing COX-2 help control the process of angiogenesis and secrete inflammatory prostaglandins that inhibit apoptosis or increase invasiveness (28, 29). Some studies have described that some COX-2 inhibitors prevent adenoma recurrence in patients with a history of primary adenoma. They have also observed that COX-2 expression is increased in colorectal adenomas and carcinomas. All these observations suggest that there is an important role for COX-2 overexpression in colorectal carcinogenesis (12, 30, 31).
However, the role of COX-2 overexpression in understanding the biological behavior of CRC is still controversial. Since Eberhart et al. first demonstrated increased COX-2 expression in CRC (32), several studies have since shown consistent COX-2 expression levels in CRC (14, 26, 27). These discrepancies in results may be related to the diagnostic methods used, the patient groups, and especially to the grading system used in the studies (33-35).
The findings of the present study showed that Ch/Cr-NPS reduced COX-2 gene expression, which is consistent with the findings of some previous similar studies. In studies conducted by Kang et al. (36), Chun et al. (37) and Hong et al. (38), it was reported that curcumin-treated cells showed reduced COX-2 expression in various cell lines by inhibiting the mitogen-activated protein kinase (MAPK) signaling pathways. Curcumin is a yellow tautomeric compound and is relatively soluble in organic solvents such as dimethyl sulfoxide, ethanol, methanol, chloroform, or acetone. Curcumin, chemically known as diferuloyl methane, is a hydrophobic polyphenol obtained from the rhizome of the turmeric plant and has a unique place in traditional therapies. The anticancer potential of curcumin stems from its ability to suppress the proliferation of a wide range of tumor cells and downregulate the transcription factors AP_1, NF_KB, and Egr_1. Curcumin inhibits protein kinase and serine/threonine protein activity by reducing the expression of NOS, LOX, COX-2, TNF, UPA, MMP9, chemokines, cell surface adhesion molecules, and cyclin D, as well as by downregulating growth factor receptors such as EGFR and HER2, and can suppress the initiation of tumor progression and metastasis (15-18, 22-24). Curcumin is pharmacologically safe; however, a major obstacle is its poor aqueous solubility, which significantly limits its bioavailability and clinical efficacy in biological systems. Therefore, considering the potential therapeutic efficacy of curcumin, efforts have been made to increase its solubility and bioavailability (39).
Chitosan, as a biopolymer with abundant resources, easy modification, and nontoxicity, can serve as an ideal carrier for the delivery of anticancer drugs. Modification of chitosan through the involvement of its amino, acetamide, and hydroxy groups can increase its solubility and exert significant anticancer activity. Chitosan and its various derivatives selectively penetrate cancer cell membranes, and its anticancer activity is mediated through various pathways, including cellular enzymatic pathways, antiangiogenic, immunomodulatory, antioxidant defense mechanisms, and apoptosis (40-42).
5.1. Conclusions
Based on the findings of the present study, it can be concluded that COX-2 expression was significantly higher in CRC tissues compared to healthy colon tissues. In addition, the Ch/Cr-NPS combination has the ability to reduce COX-2 gene expression and can be proposed as a complementary therapy in the control of CRC. This study can be an effective step towards the development of novel nanocomposite-based therapies for the treatment of gastrointestinal cancers.
5.2. Limitations
The findings of the present study are the results of an animal study and for its application to humans, more similar studies need to be conducted in the future. The fact that Ch/Cr-NPS was not compared with other effective substances for reducing COX-2 gene expression was one of the limitations of the present study.