1. Background
crystal violet (CV) is a water-soluble cationic dye that is extensively used in dyeing industries for applications such as bio-staining, medicine, disinfection, foods (1). Large volumes of effluents produced in industrial processes can enter the environment as a result of widely using cationic dyes such as CV (2). The toxicity of these dyes is normally higher than that of anionic dyes. CV is extensively used in industries and, can enter the environment in large quantities through sewage. Using appropriate systems to safely dispose of this substance therefore appears essential (3). Over the years, CV has been proposed to be eliminated using different methods, including oxidative degradation (4), electrochemical techniques (5), membrane separation (6) and biochemical absorption and degradation (7). Among different refining processes, AOPs are very important in terms of decolorization and mineralization of organic compounds through generating hydroxyl radicals. Fenton is a cost-effective, safe, fast, highly-oxidative, non-energy intensive, simple and easy to operate and maintain (8) AOP that produces no byproducts. The main advantages of the Fenton process include combining oxidation and coagulation, which results in producing less sludge compared to that produced in coagulation and flocculation (9). In addition to eliminating organic matters (10), Fenton can be used for removing certain toxic elements (8) and for the pre-treatment of biological processes (11). The Fenton reaction involves several sequential reaction steps, which are described as follows (12):
(1) H2O → •OH + H+ + e
(2) O2 + 2H+ + 2e → H2O2
(3) Fe2+ + H2O2 → Fe(OH)2+ + •OH → Fe3+ + OH + H2O
(4) Fe3+ + H2O2 → Fe–OOH2+ + H+
(5) Fe–OOH2+ → Fe2+ + HO2•
(6) H2O2 → HO2• + H+ + e
The drawbacks of the Fenton process include the risk of storing and transporting hydrogen peroxide, dealing with high amounts of chemicals and the need for neutralization before disposing of wastewater (13); nevertheless, many researchers have reported the successful application of the Fenton process for colored wastewater and organic materials; for instance, Chen et al. used this process to eliminate surfactants, and achieved a removal efficiency of over 75% (11). Perkowski et al. used the Fenton process to treat high-density detergent wastewater with a removal efficiency of 65% - 97% (14). Meric et al. reported the elimination of over 99% of the reactive dye (BLACK 5) at a concentration of 200 mg/L (15). Biglari et al. showed that the Fenton process could be used to treat water containing organic soluble carbon (16).
2. Objectives
The present article seeks to investigate the catalytic decomposition of CV in aqueous solutions using the Fenton process.
3. Methods
3.1. Materials
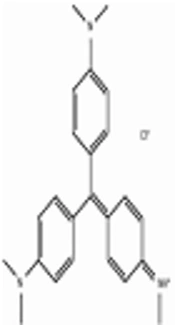
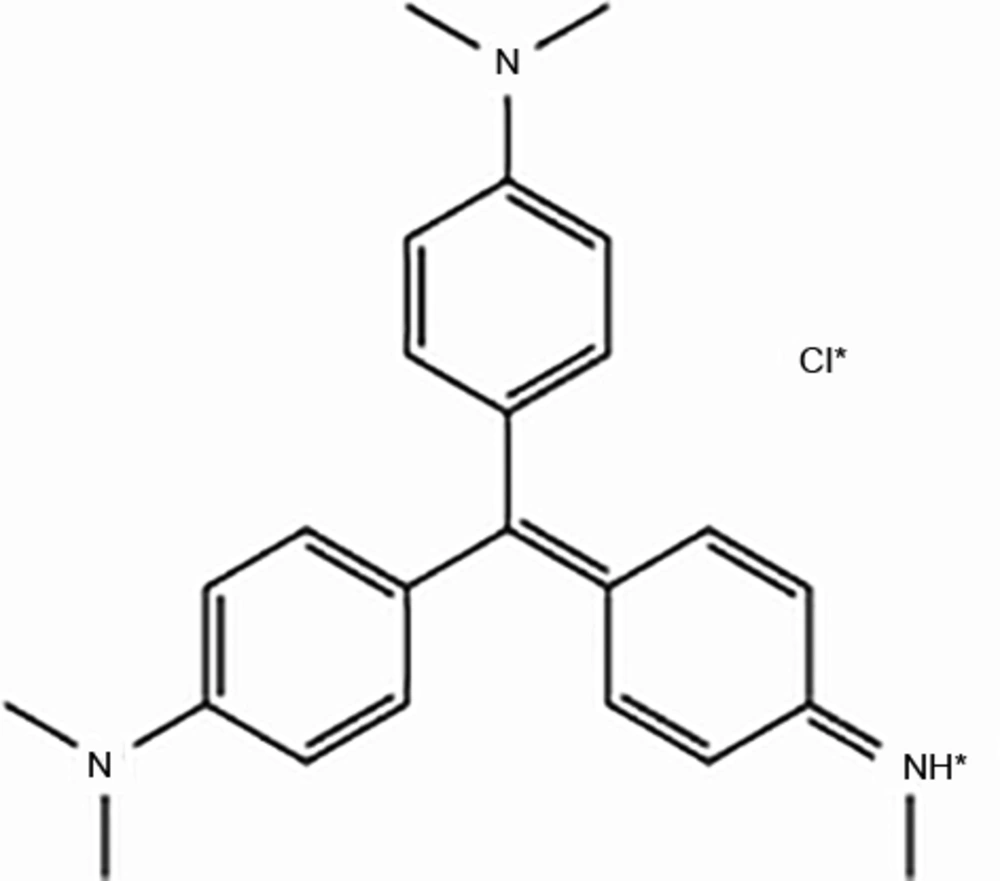
CV is a trinyl-phenyl methane dye with a molecular formula of C24H28N3Cl and a molecular weight of 396.96 g/mol. Figure 1 shows the chemical structure of CV.
3.2. Setup
A laboratory scale and a batch mode were designed for the decolorization of CV. A Pyrex glass cylinder with an effective volume 500 ml was used as the chemical reactor. A dye stock was prepared with an approximate 100 mg/L concentration of CV by dissolving the dye aliquots into tap water. Ferrous ions and hydrogen peroxide were used at a 1:10 ratio both with a concentration of 1 mM. To adjust the sample pH, 1 M NaOH and 1 M H2SO4 were used in a WTW pH meter. A 100-rpm magnetic stirrer was also used to ensure the homogeneity of the solution. The maximum wavelength of CV was determined as 586 nm using a UV-VS spectrophotometer (Jenway 6305). Data analysis and interpretation was performed in Excel 2010 (version 14.0).
4. Results and Discussion
4.1. Fe+2/H2O2
Pollutants can be generally decomposed in the presence of iron ions and hydrogen peroxide owing to the formation of hydroxyl radical species, should the molar ratio be correctly selected. The initially-tested ratios of Fe+2/H2O2 included 1:10, 1:5 and 1:1. The optimal ratio of Fe+2/H2O2 maximizes the number of oxidative radicals produced. In other words, suboptimal doses of hydrogen peroxide act as a radical scavenger, and ultimately reduce the oxidation efficiency. In addition, high doses of hydrogen peroxide are practically used for the pre-disposal treatment of wastewater and deal with potential biological processes. The optimal doses of hydrogen peroxide and iron ions should be therefore obtained from laboratory studies. According to the results (data not shown), the optimal ratio was found to be 1:10.
4.2. Effect of pH
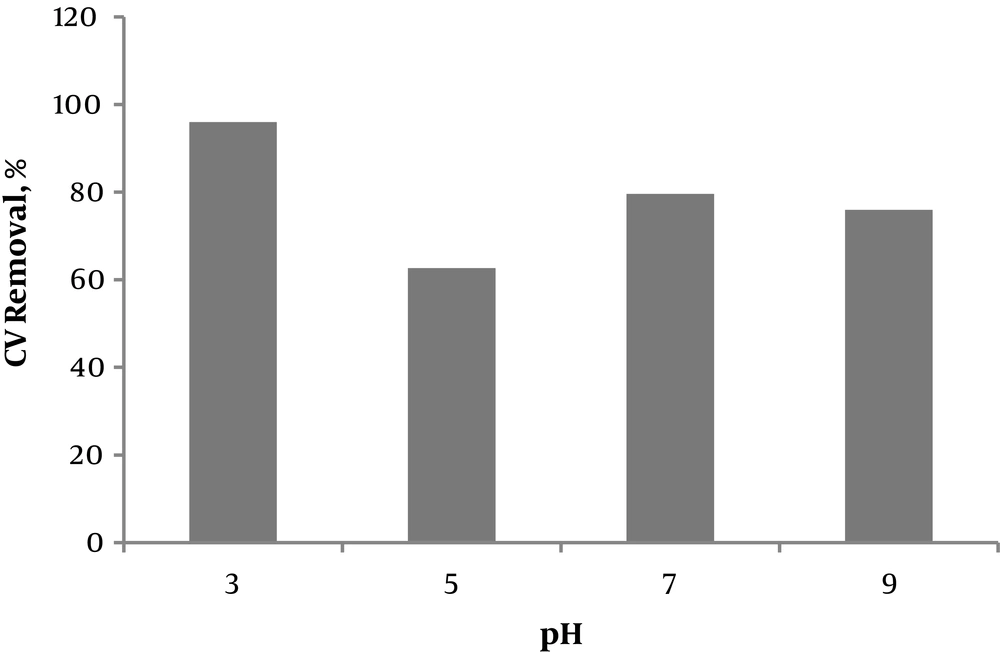
pH is an effective parameter in many chemical reactions. The optimal pH was determined by investigating its effect on the Fenton process through evaluating pH 3 and 5 as the indicator of the acidic character, pH 7 of the neutral character and pH 9 of the alkaline character. This test was conducted for 30 minutes at a CV concentration of 20 mg/L, a stirring speed of 100 rpm and an Fe+2/H2O2 ratio of 1:10. Figure 2 shows the effect of different pH values on the efficiency of the Fenton process in eliminating CV, suggesting that pH 3 is the optimal with an elimination efficiency of 96.1%. The catalytic activity of iron species highly depends on the solution pH. The concentration of divalent iron species and therefore the rate of the Fenton reaction and the decolorization percentage are maximized at pH 3. Moreover, at pH values over 5, trivalent iron species precipitate as Fe(OH)3 molecules, reducing the amount of catalyst in the solution. The amount of available free iron ions and hydroxyl radicals are reduced at higher pH values due to the deposition of Iron ions as Fe(OH)3 molecules. In other words, hydrogen peroxide is spontaneously decomposed at higher pH values.
A review of literature suggests that, at a pH below 3, the complex species of [Fe(H2O)6]2+ are formed and their reaction with hydrogen peroxide is much slower than that of other species, which lowers the efficiency. At relatively high concentrations of H+, i.e. a strong acidic pH, hydrogen peroxide is dissolved and forms stable oxonium ions of H3O2- (17). Given that the reactivity of oxonium ions with iron ions is much lower than that of hydrogen peroxide, the efficacy of the Fenton process in decomposing organic compounds is reduced at a very low or high pH, and the optimal pH of the Fenton process is 3.
4.3. Initial Concentration of Dyestuff
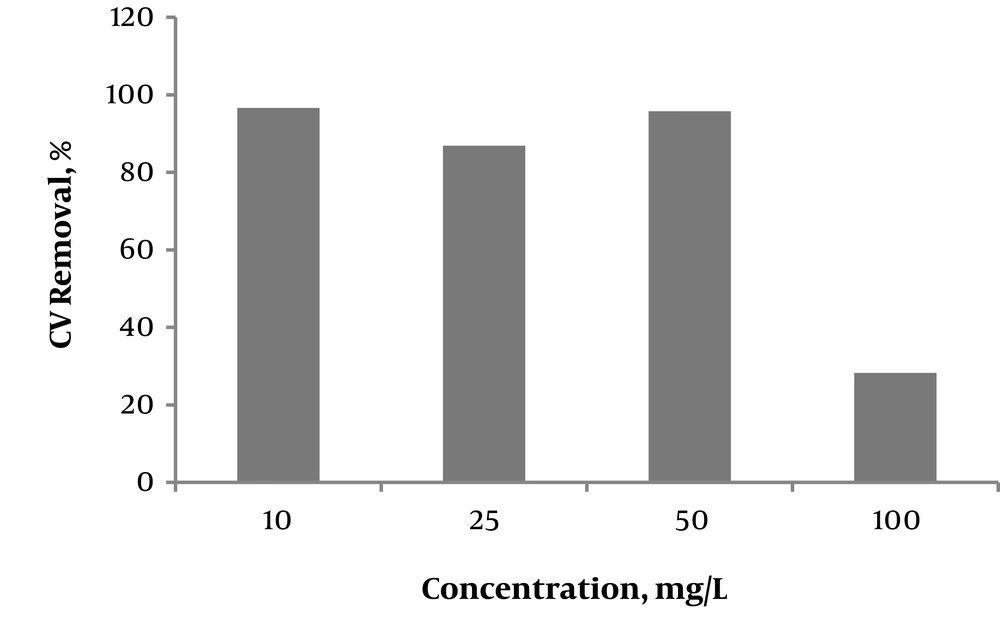
To determine the effect of dye concentration, 10, 25, 50 and 100 mg/L of CV were prepared. Figure 3 shows the effect of the initial concentration of the dyestuff on the Fenton process, suggesting a decrease in the efficiency of removing CV with increasing its concentration from 50 mg/L to 100 mg/L. At lower concentrations, especially below 50 mg/L, decolorization was successful with a minimum removal percentage of 86% - 96%. The Fenton process was therefore found to be more efficient at lower concentrations of the dye. These results suggest that dilution can be helpful or even necessary before performing the Fenton process. This finding can be explained by the fact that the decolorization of dye molecules is limited by the number of radicals and the contact duration. In other words, in lower concentration of dye, better decolorization performance is happened.
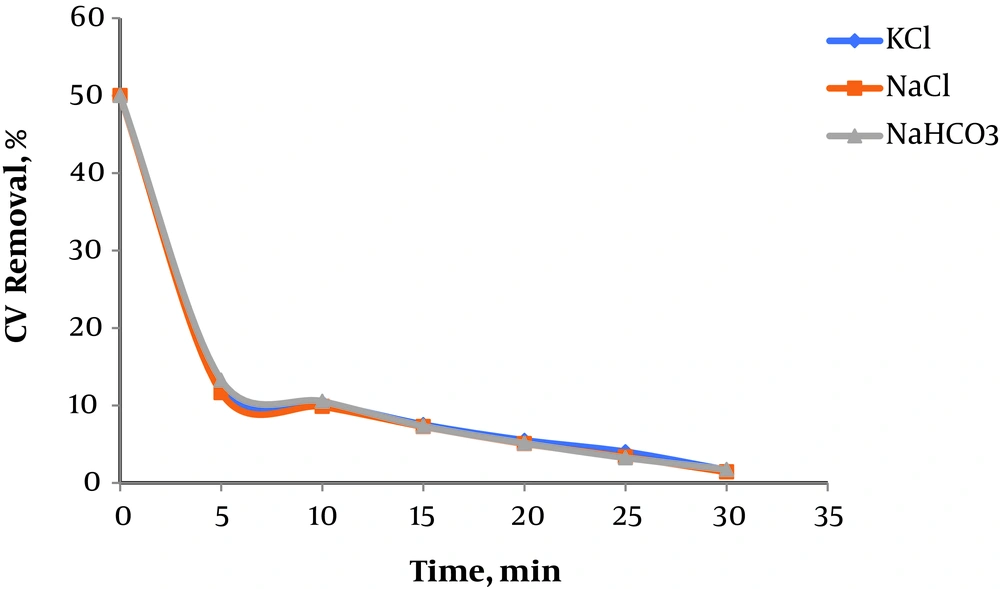
4.4. Effect of Interfering Salts
The effect of interfering salts such as KCl, NaCl and NaHCO3 were evaluated at this stage. This experiment was performed at a pH of 3, a Fe+2/H2O2 ratio of 1:10, an initial concentration of 50 mg/L and a 0.1 g/L concentration of the interference. Figure 4 shows the effect of different salts on the efficiency of the Fenton process in removing CV, suggesting the significant impact of the interference, which is potentially associated with the relatively low concentration of the interference, i.e. about 0.1 g/L. The Fenton process can be therefore successfully accomplished in the presence of low levels of interfering salts. Research suggests that NaHCO3 exerts a significant scavenging effect on hydroxyl radicals. The bicarbonate ions can take the interfering role in the following reaction (18).
4.5. Literature Review
Table 1 summarizes the studies in which different dyes and pigments were degraded with Fenton and Fenton-based processes, suggesting that Fenton is a strong process for the degradation/decolorization of a wide range of dyes. Decolorization was completed in some cases within a few hours of operation. Research also suggests a dye mineralization efficiency of over 80% (19, 20).
| Process | Dye | Wastewater | Experimental Condition | Efficiency | Reference |
|---|---|---|---|---|---|
| Fenton type: Fe2+/H2O2, Fe0/H2O2, photo-Fenton based: UV/ Fe2+/H2O2, UV/ Fe0/H2O2 | Acid Orange 7 | Synthetic | Optimal: pH 3, Fe2+/H2O2 1:5 - 50, duration < 10 min, dye concentration: 20 mg/L | > 94% | (21) |
| Electro-Fenton | Azobenzene | Synthetic | pH 2 - 3, 0.5 mM ferric iron, Current: 60 mA | mineralization yield 80% | (19) |
| Fenton, Fenton-like | CV | Synthetic | Optimal: pH: 3, 30 min, dye concentration: 0.15 mM, Fe2+/H2O2 0.5:50, Fe2+/H2O2 1:50 | ~100% | (22) |
| Fenton (H2O2/Fe2+) and photo-Fenton (H2O2/Fe2+/UV) | Reactive Black 5 | Synthetic | Optimal: [H2O2]0/[RB5]0 4.9:1, [H2O2]0/[Fe2+]0 9.6:1, pH 3 | 97.5% 98.1% | (23) |
| Heterogeneous Fenton-like | Orange II | Synthetic | Optimal: pH: 3, Catalyst: 0.2 g, H2O2 concentration: 6 mM and temperature: 30°C | Mineralization 90% | (20) |
| Electro-Fenton | Alizarin red | Synthetic | Na2SO4 0.05 M, pH 3, T 25 °C, Fe2+ 1 mM, Applied current: 200 mA, 120 min | ~100% | (24) |
| Fenton | CV | Synthetic | Optimal: pH 3, Duration: 30 min, Fe2+/H2O2 1:10, Dye concentration: 50 mg/L | > 96% | Present study |
5. Conclusions
The present study determined the efficiency of the Fenton process in the decorolrization of CV in an aqueous matrix. The optimal conditions included pH 3, an initial concentration of 50 mg/L and an Fe/H2O2 ratio of 1:10. The obtained results suggest that the Fenton process can be used for the decolorization of colored wastewater.