1. Background
Polycystic ovary syndrome (PCOS) is the most common endocrine disease in women (1). PCOS is a form of hyperandrogenic ovarian function (1). The disorder in androgen production by the ovary emerges during puberty, but roots in childhood or even in the fetal period (1). PCOS is one of the most common disorders affecting 6% - 8% of women in reproductive age and is the most common cause (almost 75%) of infertility (due to anovulation) (1).
In addition to infertility, PCOS is associated with insulin resistance, hyperandrogenism, metabolic syndrome, and an increased risk for diabetes (2). Women with PCOS have higher levels of testosterone, insulin, cholesterol and triglycerides than healthy people (3). Moreover, women with PCOS have lower levels of follicle stimulating hormone (FSH), sex hormone - binding globulin (SHBG) and high-density lipoprotein than healthy people (3-5). Recently, adipose tissue has been recognized not only as a storage organ of excess energy but also as an active hormone system in metabolism control. This tissue produces and secretes a number of biologically active proteins such as enzymes (6). Adiposity is expressed by the Complement Factor D (CFD) gene on chromosome 10. The adipose gene is asymmetric with respect to the location of diabetes (chromosome 4) or obesity (chromosome 6) gene. Therefore, changes in adipose concentration in these diseases are due to regulatory deficiency (7).
Adipose tissue is involved in various biological processes including blood clotting, activation of supplements, fertility, immune system, development and repair of tissues, blood pressure, body weight, absorption of nutrients, fibrinolysis, cell proliferation, bone formation and apoptosis (8). Adipose tissue plays an important role in the host defense system as a rate-limiting enzyme in the alternative complement pathway. Adipose tissue also activates the substitute complementary pathway called complement factor D, which is a rate-limiting enzyme in the complementary system. The complementary system is part of the intrinsic immune system. Therefore, adipose tissue is a major component of the immune system, in addition to its role in metabolism (9). High concentrations of adipose are present in adipose tissue, which indicates that complementary activation occurs in adipose tissue (10). The important role of adipose in diseases such as obesity and diabetes has recently been identified, but its function has not yet been fully understood (10). Clinical studies have shown reduced levels of adipose in several animal models of obesity (11) while human studies have reported that increased serum levels of adipose in metabolic diseases are associated with body mass index (BMI) in obese people, menopausal women with metabolic syndrome and obese pregnant women (12, 13). Although the interaction between the complement system and the adipose tissue has been identified, the pathophysiological significance of this interaction has not yet been clarified. There is increasing evidence that there is an interface loop among the complement system and inflammation, obesity, insulin resistance, and cardiovascular disease (13). There is some evidence that there is a connection between the Complement system and the PCOS (14). Gursoy Calan et al. (15) enrolled women with PCOS in a cross-sectional study and reported high adipose levels in them. They reported a significant positive correlation between adipose and variables of BMI, insulin resistance (evaluated by homeostasis model), free testosterone, C-reactive protein with high sensitivity and carotid intima media thickness. In contrast, Hashemi et al. (16) did not report any significant correlation between serum adipose and PCOS, but reported that glucose and insulin levels were high in people with PCOS and they had insulin resistance. It is known that adipose concentrations change in obesity and insulin resistance, a direct relationship may exist among obesity, insulin resistance and PCOS, women with PCOS are reported to have high levels of adipose, and there is a relationship between adipose and VAI.
2. Objectives
The present study sought to assess the incidence of adipose changes and VAI following a 12-week program of intense interval in women with PCOS.
3. Methods
The present study is a quasi-experimental design with pre-test and post-test implemented in a field form. The statistical population in this study included patients with PCOS in Kermanshah city presenting to Imam Reza Hospital affiliated to Kermanshah University of Medical Sciences. Previously, the disease was diagnosed and confirmed by an endocrinologist through clinical, biochemical, and sonographic tests on the basis of criteria in Rotterdam (17). Exclusion criteria were cigarette smoking, infection and any medication effective on laboratory results. Among eligible patients, 24 patients (according to semi-experimental study, similar studies with exercise intervention) with a range of 20 - 40 years old who volunteered to collaborate, were selected by convenience sampling method. Patients were randomly divided into two groups: aerobic training (12 patients) and control (12 patients). Random number table was used for randomization.
The exercise group participated in a daily supervised home aerobic exercise program, but the control group did not receive any intervention and their normal living process continued throughout the study. The training program in this study was individual and daily. The goal was to achieve specific exercise energy consumption at each session. The specific exercise energy consumed during the first four weeks was equivalent to 4% of the estimated individual energy requirement for weight gain, to 6% during the fifth to eighth weeks, and to 8% during the 9th to 12th weeks. The required energy was also derived from the published equation for the energy required to maintain the weight obtained at the Pennington Research Center for those with normal and ordinary lives (18):
Energy requirement (kilocalories per day) = 1625 + 31.8 (fat free mass in kilograms) + 1.5 (fat mass in kilograms) - 187 (for females)
After calculating the specific exercise energy (ExEE) for each session and converting it to the amount of active oxygen consumed, this value is set by the American College of Sports Medicine formula for stepping calculus (with a height of 25/16 inches or 41/275 cm of steps and 22 steps per minute intended for the Queens College step test (devised by McArdle et al. in 1972), the stepping time required to achieve energy consumption is calculated. Was obtained:
VO2maxmL.kg-1.min-1 = (0.2 × f) + (1.33 × 1.8 × f × h) + 3.5 mL.kg-1.min-1
After specifying the time required for stepping on each individual and presenting it in the special table for consecutive weeks with increased energy intake, the study patients were asked to complete their stepping program every day at eleven o’clock in the morning (Rhythm 88 acoustic signal per minute for quadriceps with 22 rhythms per minute studied in a group file on a mobile phone). To monitor and ensure that the exercise program was performed correctly, daily and randomly, the research team visited 5 patients at home and supervised their exercise training.
3.1. Training Program
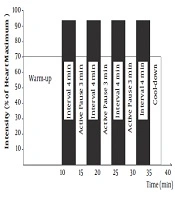
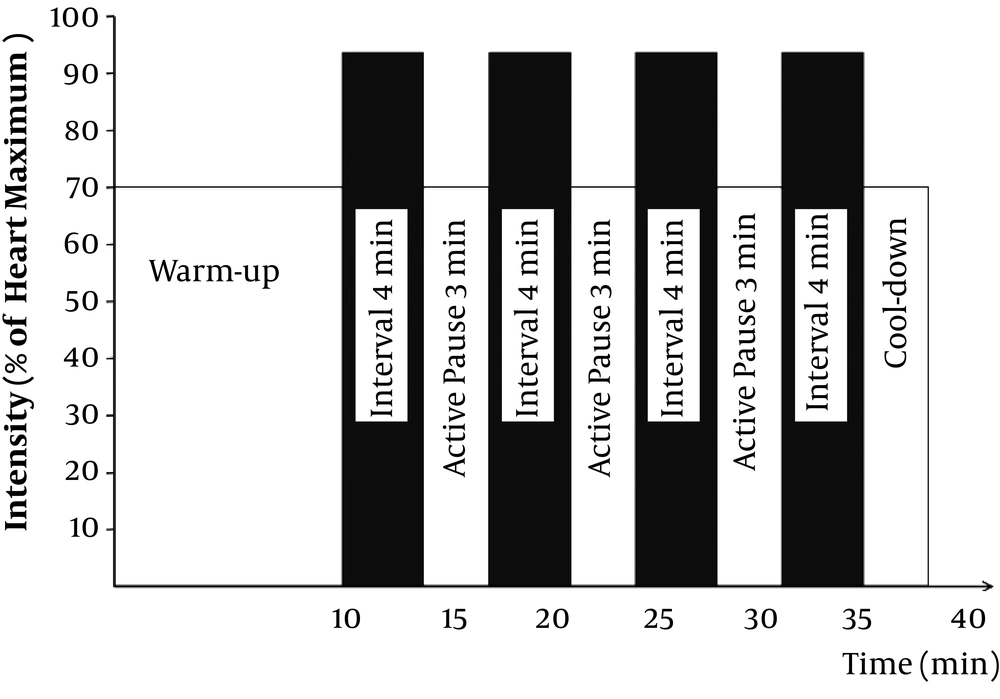
A severe training program was conducted for 12 weeks and three sessions per week. Two sessions of intense interval training per week consisted of four-minute exercises with 90% to 95% of the maximum heart rate, separated by the three exercises with moderate intensity of 70% of the maximum heart rate (Figure 1).
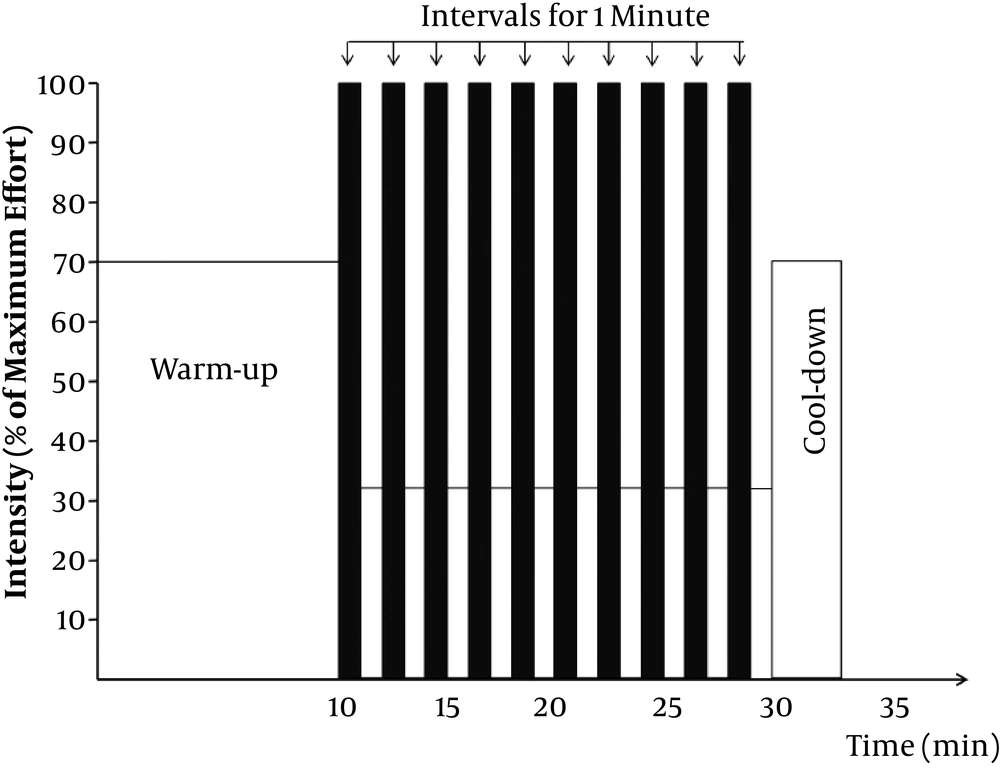
A weekly exercise session included exercises with maximum intensity and full force separated by very low activity or one-minute rest (Figure 2).
3.1.1. Blood Biochemical Analysis
Blood samples were obtained 24 hours before the first training session and 48 hours after the last training session (after the exercise protocol) for all patients after at least 12 hours fasting to check the biochemical variables (VAI, adipose level), in laboratory conditions, about 3 cc blood samples were collected from the anterior cubital vein of patients. Sampling was performed at a specific time of day (8 to 10 am) so that VAI and adipose levels were not affected by their circadian fluctuations. Blood samples were centrifuged for 15 minutes at a rate of 3000 rpm and frozen at -80ºC.
Serum adipose level was determined by Sandwich ELISA using a human adipose ELISA kit (Isotope, a Japanese student feather company) with a sensitivity of 0.39 pg/mL. Also, VAI levels were evaluated by an enzyme-linked immunoturbidometric assay.
Lipid indices including total blood cholesterol, HDL cholesterol and triglyceride (TG) were measured using special kits for clinical laboratory (Pars Azmoon, Iran).
3.2. Body Mass Index Evaluation
Weight was measured with a digital scale (Seca Company) with light clothes and barefoot, and height was measured with a fixed tape meter mounted on the wall in a standing position and barefoot while the shoulder and heels touched the wall. Body mass index (BMI) was calculated by dividing weight (kg)/ height2 (m).
3.3. Visceral Adiposity Index Evaluation
Based on the linear equation between body mass index [kg/m2] and waist circumference (WC) [cm], a model has been proposed for fat tissue distribution (MOAD) that has a strong correlation with visceral adiposity mass determined by MRI. This model was later reformed for modified TG and HDL (mmol/L) values and visceral adiposity index as follows:
3.4. Analysis
Data was analyzed in SPSS software (version 22) using descriptive statistics of mean and standard deviation, and inferential statistics of Kolmogorov-Smirnov test to investigate the normal distribution of data. Dependent t-test and independent t-test were used to examine the differences between groups at a significance level of 0.05.
4. Results
Table 1 shows the descriptive characteristics of the patients and homogeneity of the intervention and control groups.
| Variable | Mean ± SD | t | P |
|---|---|---|---|
| Age, y | 0.957 | 0.348 | |
| Control | 32.18 ± 3.47 | ||
| Intervention | 34.34 ± 4.69 | ||
| Height, cm | 0.495 | 0.625 | |
| Control | 173.48 ± 6.27 | ||
| Intervention | 176.71 ± 5.39 | ||
| Weight, kg | 0.202 | 0.241 | |
| Control | 63.24 ± 8.56 | ||
| Intervention | 67.97 ± 6.41 | ||
| BMI, kg/m2 | 0.823 | 0.419 | |
| Control | 21.33 ± 2.62 | ||
| Intervention | 21.19 ± 2.62 |
As shown in Table 1, there is no significant difference between the two groups, and the two groups matched in terms of age, weight, height, and BMI.
Adipose level and VAI in the control and intervention groups before and after intensive interval training are shown in Table 2.
| Variable | Pre-Test, Mean ± SD | Post-Test, Mean ± SD | t | P |
|---|---|---|---|---|
| Control | ||||
| Adipose (ng/dL) | 14.46 ± 4.51 | 14.62 ± 4.33 | 1.438 | 0.178 |
| Visceral adiposity Index (VAI) | 35.74 ± 8.62 | 35.43 ± 7.89 | 1.39 | 0.192 |
| Intervention | ||||
| Adipose (ng/dL) | 15.38 ± 4.09 | 15.75 ± 4.33 | 3.171 | 0.089 |
| Visceral adiposity Index (VAI) | 33.37 ± 8.28 | 30.11 ± 7.62 | 2.273 | 0.044* |
As shown in Table 3, dependent indices (adipose, VAI) did not change significantly in the control group, but adipose increased in the intervention group after the intervention. But decreasing has been observed in other measured variables.
| Variable | Group | Variation (%) | t | P |
|---|---|---|---|---|
| Adipose | Control | 1.1 | 1.438 | 0.178 |
| Intervention | 2.4 | 3.171 | 0.089 |
The results of paired t-test showed no significant difference in intra-group changes in adipose levels in the intervention (P = 0.089) and control group (P = 0.178), which indicates that intense interval training did not affect adipose levels in women with PCOS (Table 3).
Independent t-test showed no significant difference between changes in adipose levels between control and experimental groups (Table 4). This indicated that the effect of exercise on adipose levels was not significant in the experimental group.
| Variable | Pre-Test | Post-Test | ||
|---|---|---|---|---|
| t | P | t | P | |
| Adipose | 1.113 | 0.227 | 0.515 | 0.611 |
Paired t-test showed a significant difference in intra-group variation of VAI in the experimental group (P = 0.44). However, there was no significant difference in the control group, indicating the effect of interval training on serum VAI in women with PCOS (Table 5).
| Variable | Group | Variation | t | P |
|---|---|---|---|---|
| VAI | Control | 0.86 | 1.39 | 0.192 |
| Intervention | 9.76 | 2.273 | 0.044* |
The results of independent t-test showed a significant change in VAI between control and intervention groups, which indicates the effect of training on improving the level of VAI in women with PCOS (Table 6).
| Variable | Pre-Test | Post-Test | ||
|---|---|---|---|---|
| t | P | t | P | |
| VAI | 0.471 | 0.642 | 2.807 | 0.01* |
5. Discussion
PCOS is the most common endocrine disorder in women of reproductive age and accounts for about 10% of women’s population. The prevalence of PCOS among Iranian women is reported to be 15.2%. The purpose of this study was to determine the effect of 12 weeks of intolerant training on adipose levels and VAI in women with PCOS presenting to Kermanshah health centers. This quasi-experimental study was performed among 24 women with PCOS in Kermanshah.
The results of the study showed that there was no significant difference between the experimental groups (P = 0.089) and adipose levels after the completion of intense training. But VAI was significantly lower in the intervention group than that in the control group (P = 0.44). The results showed that an interval of intense training did not have a significant effect on the level of adipose in women with PCOS. In a study by Traub (19), insulin levels increased in women with PCOS. The role of adiposity as a major cause of obesity and diabetes has recently been recognized, and the function of this protein is not completely determined (20). Hashemi et al. reported no significant correlation between adipose levels and insulin resistance index (16), while Mlinar et al. (21) stated that adipose levels increase in insulin resistance. Meanwhile, Xia and Cianflone (22) reported that in men, adipose levels increased in central adipose tissue and decreased in subcutaneous adipose tissue, and thus, BMI increased tend to body fat rather than fat subcutaneous, adipose levels also increase. However, in women, the level of adipose is reduced with the increase in BMI, which is likely to reduce the expression of adipose in female adipose tissue due to limiting the development of adipose tissue in women’s obesity. Serum adipose levels decreased with increasing BMI in women, and insulin levels increase with increasing insulin resistance; therefore, the lack of a difference in this group is probably due to the interventive effects of these two factors (23). Hashemi et al. (16) stated that adipose is a protein secreted from adipose tissue that controls body metabolism and adipose, including adipose, has a systemic role in lipid metabolism or other physiological systems associated with energy balance and in various studies, adipose serum levels have been associated with a change in body mass index and insulin resistance. Furthermore, the results of a study did not show a significant correlation between adipose levels and PCOS (16), which is consistent with the results of the present study. Chou et al. reported that by treating adipocytes cultured with insulin, the mRNA expression of adipose was reduced from 75% to 68% by injecting the mice with glucose (24). However, in a review study published by Mlinar et al., the adipose level increases with insulin levels under the conditions of insulin resistance in which insulin is higher than normal (21), which contrasts with the results of the present study. Adipose levels are also considered as secondary signs of obesity (18). Also, Villa and Pratley (25) reported that women with PCOS do not have an increase in VAI. Therefore, the abdominal distribution of fat cannot explain the metabolic abnormalities observed in PCOS (25). The results of this study showed a significant difference in VAI among womens with PCOS after intense interval training period. Earlier, some studies have reported the reduction of central and subcutaneous and visceral fat indices after a physical course (26, 27). For example, Yip et al. (27) reported a 31% reduction in visceral adipose and a 26% reduction in abdominal subcutaneous fat following a weight loss diet. Takami et al. (26) observed a 25.8% decrease in fat and 17.2% decrease abdominal subcutaneous fat after an aerobic training period. Exercise may reduce the waist circumference independent of changes in BMI (28). In addition, exercise, even without weight loss, reduces visceral fat mass and prevents obesity (28). Rass et al. (2000) showed that limiting calorie intake or performing the aerobic activity without limiting calorie intake is the best way to reduce obesity in patients with moderate obesity. Even exercise without weight loss is a good way to reduce visceral fat mass (29). Irwin et al. (30) also observed that about 200 minutes of exercise per week despite a modest decrease in weight resulted in a significant reduction in abdominal visceral fat in postmenopausal, housewife and overweight women. In addition, the results of this study showed that 4.2% of total body fat and 6.9% of visceral fat were decreased without limiting calorie intake (30). Kevin et al. reported that physical activity, with a significant reduction in lipid profiles, would protect individuals against the risk of cardiovascular disease. Also, Mora et al. reported that lower levels of physical activity and increased body mass index (BMI) are independently associated with an increase in total cholesterol, triglyceride and inflammatory indices such as C-reactive protein (31). Sedentary lifestyle and obesity are two main factors associated with the risk of cardiovascular disease. The risk of cardiovascular disease increases by 8% for each unit increase in BMI while it decreases by increasing physical activity (31). Studies conducted on the effect of interval training on VAI have shown that long exercises, especially over 12 weeks, have a better efficiency in reducing VAI. Furthermore, exercise training does not affect the visceral adiposity index, especially HDL, in people with a normal level of triglyceride. In other words, exercise significantly affects the visceral adiposity index (in women), which has a higher level of triglyceride and LDL (32, 33). Another reason that may justify changes in serum lipoprotein levels is the reduction in body weight, as human studies have reported that changes in lipoproteins are affected by reduced body fat mass (34). In the present study, blood lipids including triglyceride and total cholesterol decreased simultaneously with the reduction of adipocyte indices such as fat mass and body mass index, which probably reduced VAI in women with PCOS.
5.1. Conclusions
The results of the study revealed that 12 weeks of intense interval training improved and decreased the visceral adiposity index in women with PCOS, but did not affect the level of adipose in them. These findings suggest regular exercises improve the symptoms and effects of PCOS in patients. However, the effect of intense interval exercises in patients with PCOS needs more research. Considering the findings of the research and the fact that training aerobic exercise neither costs much nor requires expensive equipment, going long distances or sports venues, and also considering the role of visceral adipose and adipose indices in cardiovascular, metabolic and psychological complications, aerobic exercise can be used to improve these variables, reduce the inflammatory index, and reduce the risk of cardiovascular disease in women with PCOS. Regular physical activity has beneficial effects on the prevention and treatment of cardiovascular disease. Physical activity can have a positive effect on the cardiovascular system indirectly through several mechanisms such as increased blood volume, decreasing viscosity, increasing stroke volume, reducing blood pressure, increasing antioxidant defiance and changing blood lipids. Moreover, due to the anti-inflammatory effects of exercise, exercise plays an important role in reducing inflammatory indices in humans and can be a good way to cope with inflammatory and cardiovascular risk factors.
5.2. Limitations
Limitations of this study included not considering variables such as weight, height, BMI, diabetes, and diet.