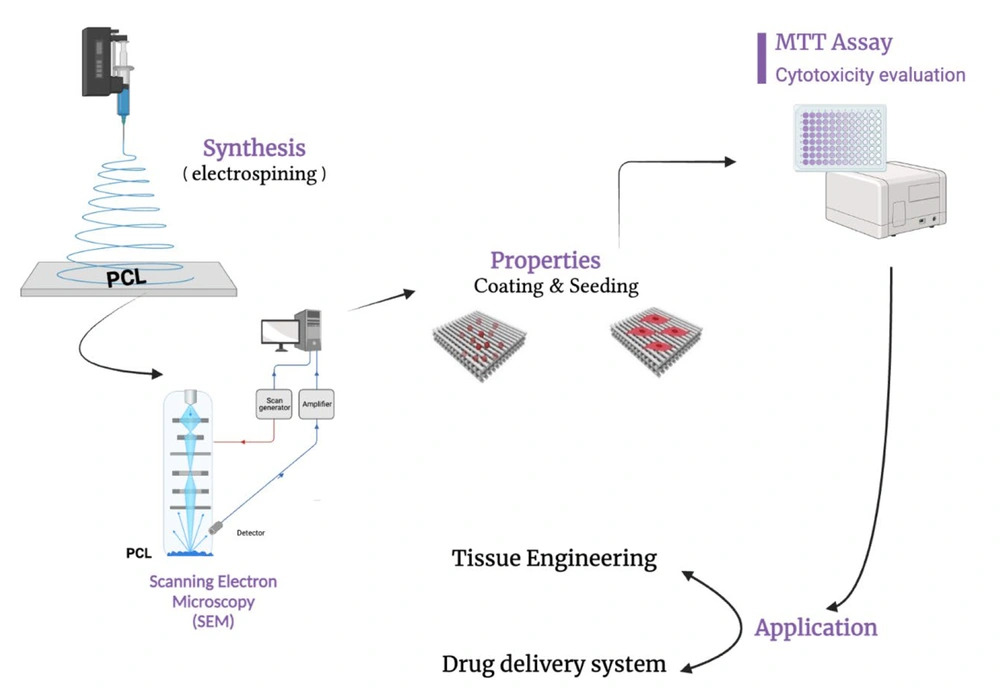
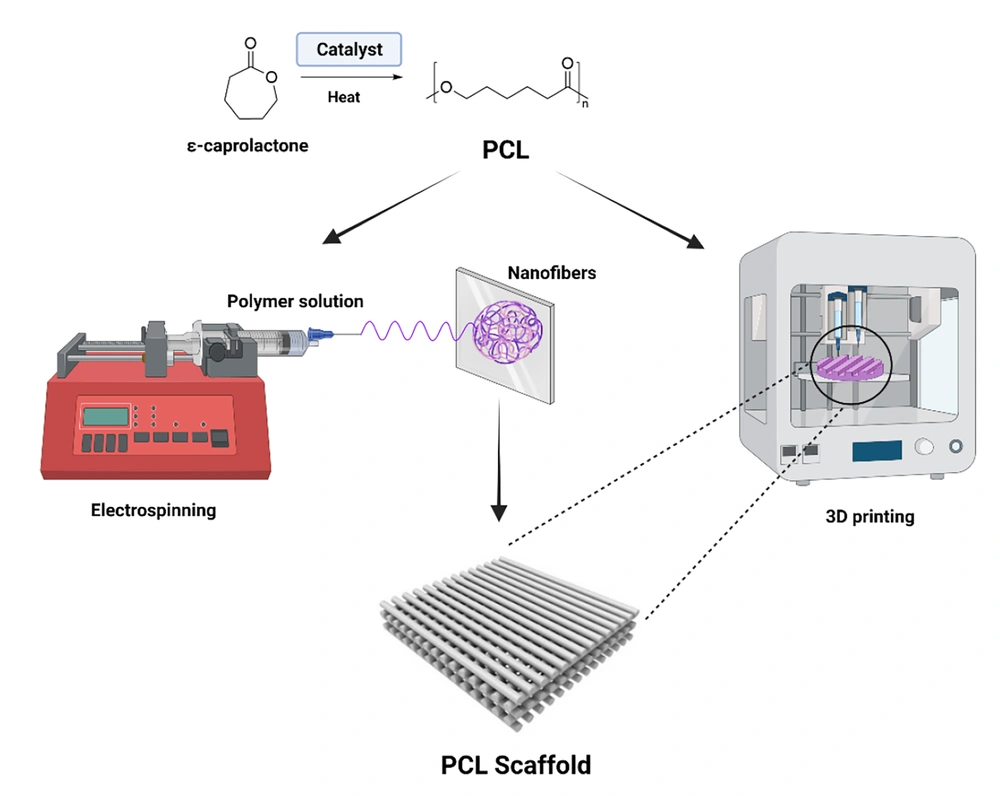
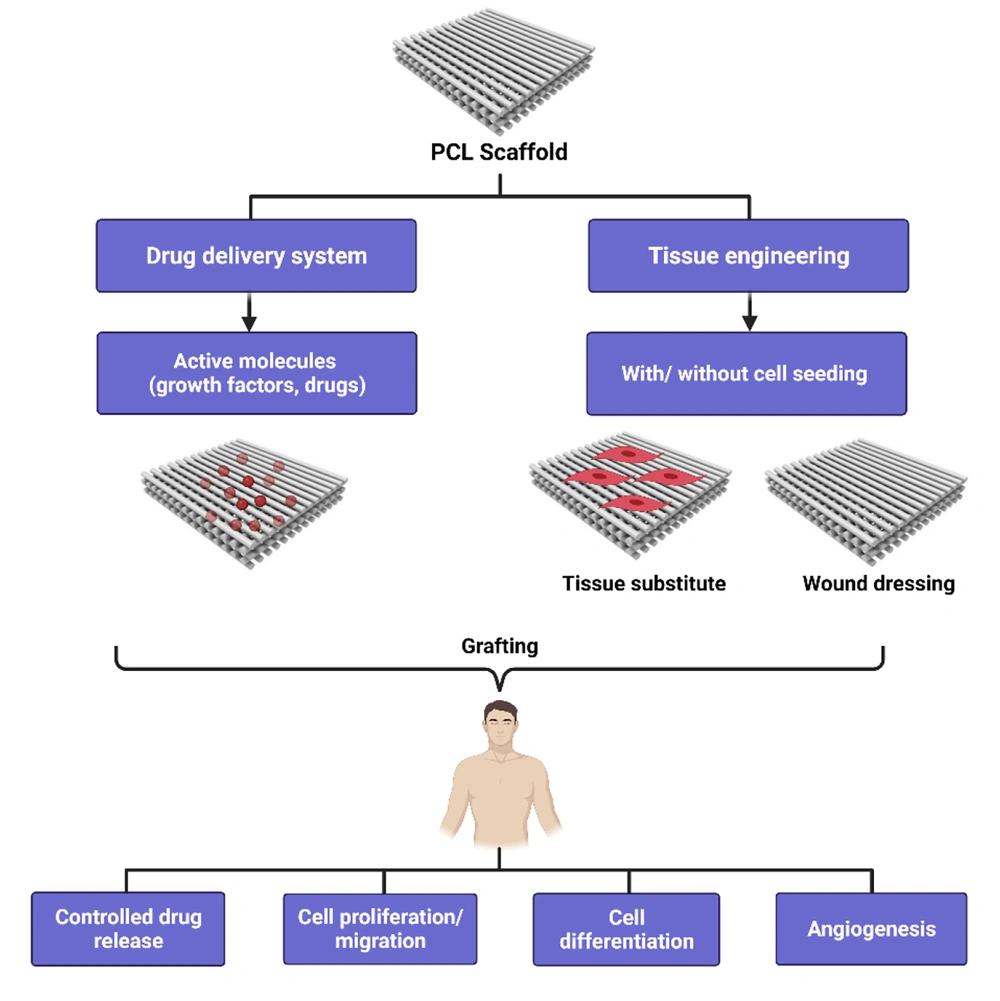
1. Context
Polycaprolactone (PCL) is an aliphatic polyester polymer with an alkaline hexanoate repeater assembly. Polycaprolactone has been closely studied with a wide variety of other polymers due to its unusual mechanical properties. Polycaprolactone's physical, thermal, and mechanical properties depend primarily on its molecular weight, degree of crystallinity, and degradation by hydrolysis of its ester bond under physiological conditions. Polycaprolactone has attracted increasing attention owing to its low melting temperature, excellent mixing compatibility, hydrophobicity, high solubility at room temperature, and simplicity of use (1). Polycaprolactone, alone and in combination with several polymers and co-polymers, has been extensively studied to prepare controlled drug delivery systems via their native biocompatibility and biodegradation. Its permeability to a wide variety of drugs has also ensured uniform delivery of drugs in the matrix, promising a lengthy release duration through a phase of decay of up to several months. Polycaprolactone has been widely used to prepare long-term implants and scaffolds that imitate the natural extracellular matrix, thereby promoting 3D cell tissue culture (2). Polycaprolactone has already been licensed for use in a wide range of medical supplies and instruments. However, few FDA and European Community Registry Label (EC) approved scaffolds containing PCL have been marketed or widely used in clinical trials (3, 4). In recent years, PCL has been used in the manufacture of green materials/biomaterials for various tissue engineering applications. Polycaprolactone's high rheological and viscoelastic properties make it suitable for the production of aliphatic polyester equivalents in a wide variety of biodegradable devices. Additionally, the mechanical characteristics of PCL complement tissue engineering applications, such as wound dressing, contraception, and dentistry (5, 6), as well as non-medical uses, such as active packaging for food products (7, 8). Polycaprolactone has been utilized in combination with other biopolymers, owing to the interesting biological characteristics of these materials. Customized kinetic and mechanical degradation are exhibited by these structures, which are readily fabricated and assembled, thereby facilitating the establishment of tissue-forming voids that can achieve precise and controlled delivery of therapeutic agents within the matrix (6, 9, 10). This example includes functional groups that change polymer chains to increase their hydrophilic, sticky, and biocompatible characteristics to induce a favorable cell response. Additionally, PCL formulations have been produced as micro-/nanomicellar structures after copolymerization, improving bioactive molecules and drug encapsulation (9, 11, 12). Owing to the increasing interest in the application of PCL as a biological and green material, we reviewed the available literature on PCL Drug Delivery and tissue engineering applications. We also provide insight into PCL safety, biodegradation, and recent developments in PCL-Based Biomaterials. In the first chapter, we focus on important synthetic techniques and show the chemical synthesis of key functional features, such as Biodegradation. In addition, the therapeutic alternatives of PCL have been discussed in a molten state or solution. Moreover, the essay concludes with a discussion of representative systems for biomedical and environmentally friendly chemical instruments (i.e., pore scaffolds, micro and nanocarriers, and implantable systems).
2. Synthesis
Polycaprolactone was developed using a caprolactone cyclic monomer ring-opener (Figure 1) and was investigated in the early 1930s (13). Recently, many catalysts have been tested for caprolactone ring-opening polymerization (14). Catalysts such as stannous octoates may be used to catalyze polymerization, and low-molecular-weight alcohols may also be used to regulate the molecular weight of the polymers. Various anionic, cationic, and directed mechanisms have been used to polymerize PCLs. The resulting molecular weight, molecular weight distribution, final group composition, and chemical structure are influenced by the phase (15, 16). Typically, the average molecular weight of PCL samples is between 3000 and 80000 g/mol and can be classified by molecular weight (17). Semi-crystalline polymers have a melting point between 59ºC and 64ºC and a transitional temperature of 60ºC in glass (18). Polycaprolactone is soluble in chloroform, dichloromethane, tetrachloride carbon, benzene, toluene, cyclohexanone, and 2-nitropropane at room temperature. It is insoluble in acetone, 2-butanone, ethyl acetate, dimethylformamide, acetonitrile as an alcoholic, oil ether, and diatomic ether (13)
Polycaprolactone is adaptable owing to its ability to be changed through copolymerization or by combining it with various polymers to exploit its electrical, chemical, and mechanical characteristics. Additionally, chemical characteristics such as crystallinity, solubility, and chain breakdown were altered, resulting in modified polymers with the expected drug transport properties (19). For tissue compositions, such as scaffolds, textiles, and films, a combination that alters the physical properties and biodegradation and strongly affects the mechanical properties is chosen. Numerous polymers have been evaluated for their ability to modify the thermal, rheological, and biophysical characteristics of PCL, according to their intended applications. Polycaprolactone has compatibility with amino polyps, hydroxyapatite, polyethylene glycol, polyethylene, polyurethane, oxazole, Polyvinyl Alcohol (PVA), and Polylactic Acid (PLA). These PCL enhancements were consistent with the biophysical characteristics anticipated for the majority of existing drug delivery formulations (17) (Figure 2).
3. Application
3.1. Drug-Delivery Systems
Polycaprolactone is used in drug delivery applications and is suitable for controlled drug release. It has superb consistency and can be excreted during resorption because of its high permeability to a range of medications. Polycaprolactone biodegradation is gradual, making it more suitable for long-term supplies of more than one year compared to other polymers. Polycaprolactone also has compatible mixtures with other polymers that can affect the degradation kinetics and rapid modifications to follow the necessary release profiles (20-22). Polycaprolactone has been modified for use in dentistry by introducing co-polymers, such as PLA or PGA, and changing the material structure with other materials, such as PEG and Ceramic (23) (Figure 3).
However, in recent drug formulations, colloidal vectors have been overlooked as effective solubilizing agents (9). Poor water solubility can limit the availability of medicinal compounds. The copolymer's micelle block can increase the number of hydrophobic molecules owing to its structural shape, which is distinguished by a sterically stable hydrophobic core. The release rate of PCL drugs depends on the formulation procedure, preparation phase, PCL quality of the medication, its size, and proportion of the drug in the microcapsules. It is combined with additional polymers to increase stress, crack, tint, and monitor opioid release speed, resulting in improved PCL permeability. In recent decades, designing controlled supply structures, particularly for peptides and proteins, has been a significant area of interest for PCL polymers (22). Lemmouchi et al. studied PCL-PLLA, PCL-DLLA, PCL-TMC, isometamidium chloride, and ethidium bromide both in vitro and in vivo (24).
The distribution of microspheres of degradable polymers has been studied extensively. Prescribing pharmaceutical drugs using these methods is beneficial because microspheres can be ingested or injected, tailored to the desired release profiles, and even organ-specific in certain situations (21). Microencapsulated medicine is a powerful delivery tool for medications with unique advantages including enhanced therapeutic safety and efficiency, improved biological activity, controlled medication release duration, and decreased administration frequency. In addition to biocompatibility, all of the most relevant requirements, such as medication particles, must biodegrade the product matrix for a long time in compliance with the drug release rate. Biodegradable polymers are also a subject of prescription test delivery system projects. After launching bioresorbable operating sutures in the market two decades ago, an extensive study on the distribution of biodegradable polymer materials was undertaken. Regarding their outstanding biocompatibility, biodegradability, and "mechanical strength," thermoplastic aliphatic polymers such as PLA, PGA, and their co-polymers in particular, poly (lactide-co-glycolides) (PLGA), have attracted considerable attention among various classes of biodegradable polymers. These structures can be easily formulated to generate antibodies, peptides, proteins, and macromolecules. In particular, the FDA has approved the supply of drugs (25). The bioresorbable matrix of microparticles can be degraded into non-toxic, low-molecular-weight organisms that ultimately metabolize and eat the host. Unsurprisingly, there is considerable scientific interest in the use of biodegradable microparticles to control medication release. Polycaprolactone has many advantages over PLA and PGAs, including high dissolution permeability for small medicinal compounds and low capability to generate an acidic environment. Compared with polyesters, the degradation of PCL homopolymers is slower, making it more appropriate for longer-term supply systems lasting more than a year, and the distribution may be increased or decreased as required with correct mixing (25). Numerous methods can be used with the PCL microspheres, which Freiberg and Zhu studied (26). In a solvent, the colloidal monomers of opposite solutions are polymerized (27). Organic monomers, such as oil in water emulsions (O/W) or water in oil emulsions (W/O), contribute to spherically distributed droplets of organic material (27). Dispersed monomers can be polymerized through various methods, including emulsions, suspensions, and dispersions. Emulsions are often used to manufacture homogeneous nanometers (10 - 100 nm). Additionally, the resultant polymer may be excessively diffractive at the nanoscale for visible light (28). The dispersion polymers have an impact of 0.5 - 10 microspheres. The reagents (monomer, initiator, and stabilizer) were dissolved in the organic medium, and polymerization occurred in the monomer droplets due to the solubility of the initiator in the monomer. Polymer pearls are insoluble in inorganic solutions and require hurrying and stabilization (29). The polymerization of supercritical CO2 dispersions has been recently studied, which may be advantageous for medical applications due to the absence of hazardous solvents (30, 31). Suspension polymerization often results in the formation of 50 - 500 microsphere meters. Suspension polymerization distributes the monomer into a water stabilizer, whereas polymerization occurs during monomer processing. The size, quantity, and velocity of the dispersed monomer droplets define their dimensions and particle quantities (27, 32). Microspheres can be rapidly produced by evaporating organic solvents from dispersed polymers and biomolecule droplets (33). Often, biomolecules are first dissolved in water and then dispersed in a bio-solvent composed of biodegradable polymers and the original W/O emulsion (usually dichloromethane, DCM). The final O/W emulsion is produced by evaporating DCM and hardening the polymers, thereby trapping the contained medicinal product (34). Successful connectivity is a major impediment in the integration of medicines into microspheres. Numerous organizations lack the pit necessary to guarantee that microspheres will continue to develop, which is prohibitively expensive. Additionally, many studies lack a clear definition of the methodology for measuring opioid prevention effectiveness.
Polycaprolactone nanosphere 5.2 Colloidal pharmaceutical delivery structures with diameters of 10 - 1000 nm are nanospheres that function as compartments for narcotics or other active molecules. Nanospheres may include encapsulated, disseminated, or swallowed medication particles. Additionally, nanoparticles or nanocapsules can be classified according to whether the medicine is enclosed in a shell or polymeric matrix. The adjustment of the processing settings for nanoscale outlets was similar to that for microparticulations. The spread and dispersion media have a modest ratio, but the riveting speed is significantly higher (35). The nanosphere may be utilized to specifically target the reticuloendothelial system of the liver and phagocytic cells. Unlike many other colloidal structures that conceal needles and capillaries, the dimensions of nanospheres enable intravenous injections. Nanoparticular-injectable carriers are well-suited for the administration of basic medicines and imaging drugs. On the other hand, the reticuloendothelial system cannot be utilized routinely since it is removed after the first few injections. To overcome this limitation, amphiphilic copolymers have been synthesized using physiologically monodispersed biodegradable nanospheres. These nanospheres demonstrated enhanced blood supply and medication concentrations in a mouse model (35). Colloidal particles and their interactions with proteins and enzymes in different bodily fluids as medication carriers are inextricably linked. The combination of lysozyme, a highly condensed and positively charged enzyme, and two drug carriers, PCL-coated nanocapsules with an oily core and PCL-shaped nanoparticles, was investigated. These findings indicated that the surface loading of lysozyme on colloidal drug carriers significantly affected the mixture (34). Gref et al. (2000) investigated the capacity of PEG-coated biodegradable nanoparticles to absorb plasma proteins and particulate matter through polymorphonuclear cells (PLA, PLGA, and PCL). This study investigated the impact of PEG corona thickness and density as well as the nature of the core (36). Freezing with many cryoprotective agents maintained the conditions required to stabilize the PLGA and PCL nanoparticles. Studies have shown that sucrose, glucose, trehalose, and gelatin additives retain nanoparticles' characteristics irrespective of the freezing process (37). Polycaprolactone has also been used as an implant for targeted drug delivery. Implants are known to exhibit high biocompatibility. A new intraocular implant using porous PCL was developed by Boia et al., which can be used to deliver dexamethasone without the need for eye drops or intravitreal injections (38).
Polycaprolactone-coated chitin-lignin fibrous gel platforms appear to be good candidates for wound dressing applications based on controlled drug release (39). Manipulating the PCL-methoxypolyethylene glycol (PCL-MPEG)-based micelles ratio is an effective approach for modulating protein adsorption, phagocytosis, and biodistribution, which may be a prerequisite for drug delivery in clinical applications (40). It has recently been shown that loading anticancer drugs on degradable PCL/magnetic nanocomposite nanoparticles effectively enhances drug function and kills cancer cells (41). Therefore, PCL is a promising polymer for pharmaceutical and biomedical applications in nanomedicine for cancer (42). Microcapsules as a controlled drug delivery carrier of Nifedipine, which is a calcium channel blocker, are widely used. Therefore, they are used to treat angina pectoris and hypertension. The core material of the microcapsules is PCL (43). Polycaprolactone nanofibers are considered carriers of oxytetracycline hydrochloride for the treatment of periodontal diseases (44).
3.2. Tissue Engineering
Usually, polymer films are formed by solvent casting, in which the polymer is molten, deposited, and evaporated from a thin film in a solvent. In Tendon, dental, skin, and Vascular tissue engineering applications, when an active surface is required to restore the patch rather than the missing tissue, biodegradable polymer films have been used (45). As they are extremely hydrophobic, films made from pure PCL do not have the requisite properties. However, the surface can be modified by shaping composites, mixtures, and copolymers to obtain the required properties. Polycaprolactone films can be used for cell binding, proliferation, and differentiation with or without external modifications. To investigate PCL’s suitability for cartilage tissue engineering, the solvent effect on the film structure was compared using acetone and chloroform dissolves (46). Prabhakar et al. compared these two groups and revealed that with few cracks, PCL chloroform-cast films had a smoother coat, better mechanical properties, and were more hydrophilic than those cast from acetone. Various film chondrogenic potentials were reported; chloroform-cast PCL films showed a higher type I/II collagen ratio with a higher chondrogenic ability and type I/II collagen ratio with a higher osteogenic capacity indication (46). Polycaprolactone cast acetone solvents were also developed by Romagnoli et al. They studied PCL film attachment and their in-vitro differentiation of mesenchymal stem cells derived from human adipose (hAD-MSCs) for use in Bone tissue engineering (47). In tissue culture, cell viability, proliferation, alkaline phosphatase activity, and calcium deposition were similar to the polystyrene plate stages, indicating PCL's affinity for hAD-MSCs. These results indicate that pure PCL films can serve as tissue engineering structures and drive stem cell differentiation without chemical changes. Chemical alteration of PCL film surfaces is one way to strengthen cell binding and enhance mechanical properties. Chemically modified PCL films for lung tissue engineering (48, 49) were prepared by Kosmala et al. Polycaprolactone was modified by aminolysis to form amine groups that connected gelatin to glutaraldehyde. Gelatin attachment can improve the viability of NCI-H292 (lung carcinoma) cells seeded on PCL. Yu et al. modified PCL films to bind a graded endothelial cell-attractive peptide on the surface. They homogenously studied endothelial cells in one direction and concluded that target cells for a specific medical application would be assisted by this type of directional cell growth (50, 51). Composite PCL-HAp films are considered safer for Bone-Tendon Recovery. Tong et al. prepared a heat-pressing composite such as PCL and nanohydroxyapatite (nHAp) (52). The addition of nHA increased the elastic modulus of the films in parallel with the number of added HA. The films demonstrated proper cell adhesion, but higher nHA content decreased ductility and separated the PCL films. Incorporating natural polymers, biological markers, or bioceramics (calcium phosphate particles) into PCL films modifies their physical, biological, and chemical characteristics and provides ample soil for cells and tissues. Polycaprolactone films can be altered by adding metallic compounds to obtain additional properties. For example, the Fe3O4 antibacterial and paramagnetic properties of skin tissue engineering (53). Pai et al. used a composite PCL film with iron oxide (Fe3O4) (53). The spin coating created a film and examined the variation in the film properties with the rotation speed of the coating process. Bactericidal effects are shown by Iron oxide particles. Because of their capacity to withstand stress cracks, PCL composites have also been employed to reconstruct bulk materials and inorganic material surfaces. Catauro et al. coated titanium zirconium-PCL solution substrates with differing dental concentrations of PCL (54). The obtained shapes were a blend of zirconium-PCL that was transformed into a film structure to protect the titanium base utilizing a dip coating. Cell viability experiments using human mesenchymal stem cells showed no significant differences and no cytotoxic impact of pure titanium. To build engineering systems for metals with antibacterial and paramagnet properties, the soft nature of PCL can be blended. Fragments of PCL on metal surfaces may also have crack resistance characteristics, which are essential for preserving material properties and improving cell-material interactions (54). Hashiwaki et al. developed mixed films using N, N-Dimethylformamide solutions for chitin butyrate-PCL (50: 50) (55). The film properties differed according to the butyryl substitution stage of chitin. Chitin with various degrees of butyryl substitution (deacetylation degree of 5 percent) was used, and high substitution levels were found, which improved its miscibility with PCL. For thermoplastic scaffolding, the mechanical strength of films can be enhanced by adjusting the chitin butyrate-PCL ratios. This system incorporated the functionalities of these two materials. The surface functional groups of PCL control its affinity and compatibility with cells. To achieve these characteristics, PCL can be modified from monomers. Chen et al. prepared PCL-coated co-polymer films with distinct functional groups and investigated their effects on physical, chemical, and biological properties (56). For this reason, ε-Caprolactone co-polymers have been prepared with modified ε-Caprolactone monomers with many functions (amine, methyl, carboxyl, hydroxyl, and aldehyde). Various functionalities of cell adhesion, such as aldehyde and hydroxyl groups, are classified as distinct biological responses. Increased osteogenesis was detected with amine-integrated films, chondrogenesis with methyl-incorporated films, and adipogenesis with the new PCL films. This means that the monomer, caprolactone, or the polymer, PCL, can be added to different functional groups, or the products can be tailored to achieve the correct cell-material interaction. Polycaprolactone copolymers with biocompatible chemicals may have a greater impact than mixtures and composites. Fuse et al. (2015) developed a film for cartilage tissue engineering using a PCL-PEG-PCL triblock copolymer casting of a chloroform/dimethylformamide mixture. The inclusion of PEG segments improved the hydrophilicity of adipose-derived stem cells in rats and resulted in rapid regeneration in a rat model of knee cartilage defects. Polycaprolactone has also been copolymerized with PLA to prepare films for application in bone tissue engineering (57). Collagen or fibronectin immobilization films were modified, and osteoblast MC3T3-3E1 cells were tested. Positive cell attachment has also been reported previously. It has been shown that engineered mesenchymal stem cells seeded on PCL nanofibrous alone or collagen-coated PCL scaffolds can be used for neural and skin tissue engineering (58, 59).
4. Biodegradation
Polycaprolactoneis destroyed under physiological conditions (as seen in the human body) to be utilized as an implantable biomaterial. Owing to its much slower degradation rate than polylactide, the development of long-term implanted devices is an intriguing prospect. Polycaprolactone degrades in two stages: First, by non-enzymatic hydrolysis of the ester groups and subsequent formation of a more crystalline polymer with a low molecular content (less than 3000) (60). This finding supports the hypothesis that PCL may be completely absorbed and removed through intracellular routes when its molecular weight is reduced to 3000 or less. Additionally, the degraded PCL was found to be almost identical to the in vitro hydrolysis at 40°C and accurate to the early stage kinetics in the first step. Polycaprolactone degradation occurs due to an unanticipated breakdown of the ester chain, resulting in a loss of molecular weight. The absolute homopolymer PCL declines during two to four years (depending on the system's weight or implant) (61, 62). Copolymerization affects the rate of hydrolysis of other lactones or glycolides/lactides. Over the past decade, over 1,000 papers on PCL-based textiles have been published in the biomass and tissue engineering literature. Polycaprolactone degradation and resorption kinetics studies were utilized (13). Improvement in the degradability of 58s glass scaffolds such as PCL by ZnO and β-TCP modification has been reported (63).
5. Current Development
The design and processing of organic plastics are gradually being studied. This was the company's exponential increase. In the timeframe up to 2020, global demand for biologically driven plastics will increase from 0.36 million to 3.45 million tons (64). In recent years, the increasing number of PCL publications has described modern biocomposites containing PCL (13, 65, 66). Polycaprolactone is a lightweight resorption polymer, particularly used in biomaterials and tissue engineering, and is popular in chemistry. Polycaprolactone can be converted into composites with better mechanical and biocompatible properties, thus diversifying its use relative to polymers. Their excellent cohesion and potential before resorbing to excretion from the PCL system are mainly used in drug delivery because of their high permeability to some drugs (67). The biodegradation of PCL is gradual and, therefore, more suitable for long-term delivery than other polymers.
It is also used in operating equipment for sutures, injured dressings, and dentistry. Tissue engineering is closely connected to tissue regeneration or repair applications in whole or part (e.g., bone, cartilage, blood vessels, and bladder). Polycaprolactone includes tissue mechanics, skeletal techniques, blood vessels, tendons, and ligaments. Over the last two decades, PCL has almost ignored the success stories of other resorbable polymers, including polylactides and polyglycolides. Polycaprolactone and its composites deliver excellent results, which translate research into future medical applications. Recently, PCL has been widely used in Tissue Engineering. The lack of bioactivity and high degradation rate of PCL have led to the investigation of its use in bone tissue engineering (23). Recent advances in PCL-based calcium phosphate ceramics have resulted in hybrid biomaterials with good mechanical properties and improved bioactivity (68). As substitutes for bone grafts, PCL and hydroxyapatite nanoparticles can be combined to form 3D scaffolds (69). A 3D-printed PCL scaffold modified with insulin-releasing PLGA nanoparticles was successfully investigated for bone tissue repair by Wei et al. The results demonstrated that this scaffold, in addition to stimulating the proliferation of chondrocytes and differentiation of BMSCs, could enhance bone and cartilage repair in vivo (70). Recent studies have shown that PCL-based composites have soft (nerve, skin, and urethra) and hard (musculoskeletal and dental) tissue-repair applications. Hu et al. prepared a degradable patch with an anti-adhesive layer using PCL, polyvinyl alcohol, and soybean peptide, which has great potential for hernia repair (71). In another study, Jeong et al. illustrated that a PCL/β-TCP scaffold could enhance bone formation in complex zygomaticomaxillary and replace traditional non-absorbable implants in the future (72). Recently, PCL-based scaffolds have attracted the attention of many researchers due to their excellent elastic properties. Several PCL-based scaffolds with excellent mechanical properties have been successfully used for wound regeneration. However, PCL applications in tissue engineering are limited by its intrinsic hydrophobic nature and slow degradation. To improve the adaptability of these scaffolds for skin tissue regeneration, PCL-based composite meshes have been developed (73). An ideal wound dressing should be biocompatible, biodegradable, antimicrobial, and removable without causing any damage to the wound (74). Polycaprolactone-based wound dressings containing Zn, Cu, and Ag exhibit enhanced antimicrobial properties and can be used for wound healing, particularly in skin infections. Muwaffak et al., who used 3D scanning to create 3D models of the ear and nose, demonstrated that PCL-based wound dressings with Cu and Ag showed excellent antimicrobial properties against Staphylococcus aureus (75). Polycaprolactone-based 3D-printed skin scaffolds incorporating Ag show promising applications in skin regeneration, as reported by Ninan et al. (76). A recent new area of PCL application concerns esthetic purposes in humans. Wee et al. introduced a three-dimensional printed PCL as an easy-to-use implant with good results for aesthetic nasal lobule correction (77). In another study, PCL-based products were used to treat facial aging by stimulating collagen formation. The long-term effectiveness and safety of PCL fillers have been confirmed in clinical studies (78).
6. Polycaprolactone Safety
Polycaprolactone systems' main advantages and functionalities have led scientists and practitioners to build and use polymers (13, 51). As explained below, particularly for the latest PCL product choices, the biomedical applications of embedded PCLs endorse PCL protection as used in the new framework (79). Polycaprolactone and polyglycolide were co-polymerized in MonocrylTM (Ethicon, Inc.; Somerville, New Jersey, USA) (80). This suture retains high tensile strength and allows the tissue to respond minimally to its defense. Polycaprolactone is ideal for long-term drug delivery owing to its elevated drug permeability, excellent biocompatibility, slow biological degradation, and bioresorbability. In addition, a specially formulated and scientifically produced levonorgestrel PCL biodegradable CapronorTM pill (up to 2 years) is used in the PCL drug delivery system, which offers valuable details on global PCL protection and supports the steady decline of PCL and its long-term safety. Polycaprolactone microspheres or nanospheres have been used in a range of medicines (62, 81-83). Various 3D-growing materials are used for bone, skin, or other tissues (19, 84), and autologous graft processing is used to repair and regenerate implantation tissue (83). Due to its physicochemical characteristics, mechanical nature, and effects, 3D printing scaffolding can also be used in the fabric manufacturing industry, an advanced research area, and an application field in which PCL plays a significant role. 3D printing uses computerized processes via subsequent layer deposition to create tissue and organ replacement structures. This is a promising treatment approach in PCL's life-saving (85-90). A Chinese team developed the first three-dimensional PCL airway for individuals who endured tracheomalacia, a life-threatening disease, and managed to save the lives of a newborn and a woman. The role of PCL in tracheal surgery has recently been explored. Congenital heart abnormalities, gastric wall injury (hollow organs), and periodontal repair have also been investigated (91-93). Orthopedic interest is enormous; for instance, meniscus repair affects millions of individuals worldwide (94-96).
7. Conclusions
Polycaprolactone has numerous applications, including in the pharmaceutical industry, such as medication release monitoring, tissue processing, and bone scaffolding. Combining PCL with other materials to form nanocomposites improves the properties of the resulting materials and expands the scope of applications that can benefit from the materials' superior qualities. This biomaterial may have potential applications in the years to come.