1. Context
Janus kinase (JAK) is a signal transducer and transcription activator involved in an intracellular communication pathway that regulates cell proliferation, differentiation, apoptosis, and immune-related processes, including inflammation, tissue repair, and autoimmune regulation (1-3). The JAK family comprises four isoforms: JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), which function as signaling mediators for other proteins, such as the signal transducer and activator of transcription (STAT) (4, 5). In mammals, STATs are expressed as seven proteins (STAT1-4, STAT5A, STAT5B, and STAT6) within the same family. Each interleukin exerts its effects through the phosphorylation of specific members of these seven proteins (6, 7). This signaling pathway is activated by necrosis factors, which utilize JAK family members to influence a range of STATs. These, in turn, regulate T lymphocytes, gamma interferon (INF-γ), natural killer (NK) cells, and the inflammatory responses of the immune system (1, 6, 8). Among the diverse array of inflammatory and necrotic factors, the JAK/STAT signaling pathway plays a critical role in the pathology of various cancers. For instance, interleukin 6 (IL-6) employs the JAK/STAT pathway in hepatocellular carcinoma (HCC), acting as a type of STAT signaling antagonist that reduces HCC immune resistance without causing harm. This mechanism has made the pathway a promising target for many chemotherapies (9, 10). Similarly, gefitinib has been shown to decrease the destruction rate of mucoepidermoid carcinoma (MCC) cells, likely by inhibiting JAK/STAT phosphorylation (11). Additionally, the breakdown of carbon ions, which reduces leukemia inhibitory factor (LIF) and subsequently diminishes STAT3 activation, has been proposed as a potential treatment for squamous cell carcinoma (12). The JAK/STAT signaling pathway also plays a significant role in regulating angiogenesis and necrosis factors in the kidney, where it is directly linked to metastasis and renal tumor growth (13, 14). Western blot analyses have demonstrated that alterations in phosphorylated STAT levels in the kidney are among the most critical changes observed in renal carcinoma (15). In this systematic review, we explore the potential for treating renal cell carcinoma (RCC) by targeting the JAK/STAT signaling pathway.
2. Objectives
This study presents RCC cell therapy based on STAT signaling as a new treatment direction. Recent studies have contributed their findings to this review, collectively investigating the therapeutic potential of this pathway in addressing one of the deadliest forms of cancer.
3. Methods
3.1. Protocol and Registration
This systematic study was conducted in accordance with the established criteria outlined in the preferred reporting items for systematic reviews and meta-analyses (PRISMA). To ensure adherence to the principle of non-bias, the Cochrane handbook for systematic reviews of Interventions (version 5.1.0) was utilized for evaluating clinical studies. The studies were rigorously assessed with respect to allocation concealment, as well as the blinding of participants, study personnel, and outcome assessors. The research question guiding this systematic study was formulated based on the PICO framework (patient/population, intervention, comparison, outcomes) that was: Is inhibiting the JAK/STAT signaling pathway a treatment for renal carcinoma?
3.2. Eligibility Criteria and Search Strategy
We assessed published studies from January 2014 - 2024 in databases such as Web of Science, PubMed, and Scopus. The definition of renal carcinoma was considered on the basis of the international classification of diseases 11 (ICD-11), and its details were adjusted on the basis of the definition of the American Cancer Society (ACS). All studies that investigated renal carcinoma on the basis of the above definition were reviewed. Studies that directly investigated JAK/STAT signaling were included in the study. Studies with the following characteristics were eliminated:
- Studies that were not open access were selected on the basis of the PRISMA checklist if the abstract was complete, and the corresponding author was contacted for the results if more information was needed. Studies whose abstract structure was not complete on the basis of these criteria were excluded from the review.
- Non-English studies were excluded.
- Studies that did not have open access and whose basic information was unavailable in the abstract.
- Studies that were based on animal investigations.
- Our research question was based on the treatment of RCC, so studies that investigated the therapeutic effect of a specific drug were not excluded from the study population.
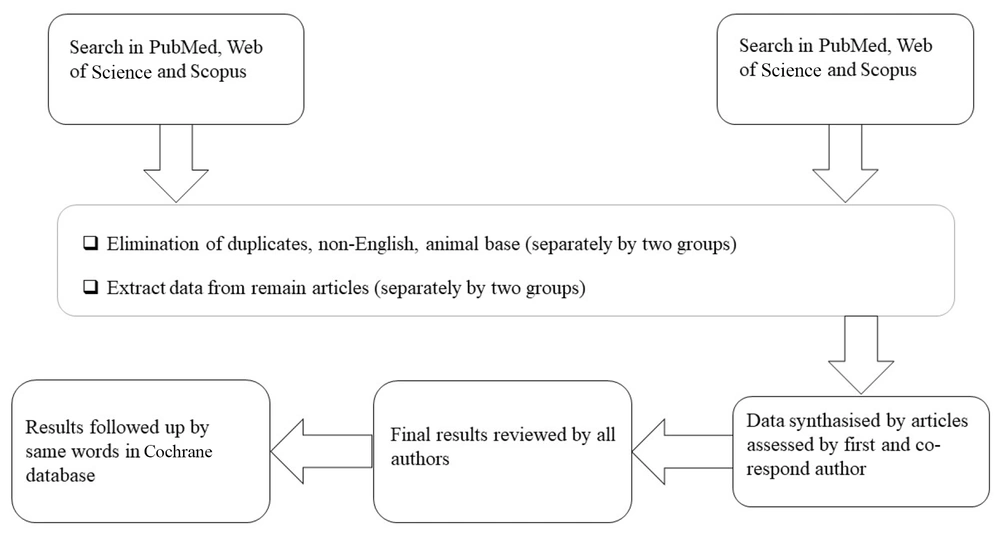
We evaluated published studies from January 2014 to 2024 using databases such as Web of Science, PubMed, and Scopus. To identify similarities and review comparable systematic studies, we conducted a title search within the Cochrane database. Keywords were derived from the MeSH database. Two teams, each consisting of two individuals, independently searched the databases, first Web of Science, followed by PubMed and Scopus, using the keywords "JAK/STAT inhibitors" and "renal carcinoma". In alignment with PRISMA criteria, duplicate, non-English, and conference entries were initially excluded. Subsequently, titles and abstracts were screened by both groups. All articles included were in vitro types and meeting the inclusion criteria were shared with the coauthor for consolidation. In cases where access to articles was restricted, the first author supervised the review process to resolve the issue. Ultimately, thirteen studies were selected, and their full texts were thoroughly evaluated. No additional articles were included through manual citation searches (Figure 1).
3.3. Data Collection and Synthesis
The corresponding author and first author formed the summary table (Appendix 1 in Supplementary File) and reviewed the studies via the Cochrane tool on risk of bias 2 (RoB 2) (16). To extract the data, a group of three authors independently examined the side signaling pathway, related immunity elements, and the JAK/STAT pathway from the articles, without communication. The results were reviewed under the supervision of the corresponding author and first author, and all the articles and results were reviewed under the supervision of the first author and second author according to the Cochrane guidelines to minimize bias error and ensure the accuracy of the information. The following information was extracted from the articles: First author, main protocol (human or laboratory), study main aim, related immunity elements, main findings, JAK/STAT type, side signaling pathway, investigation method, and inhibitors.
4. Results
4.1. Search Results
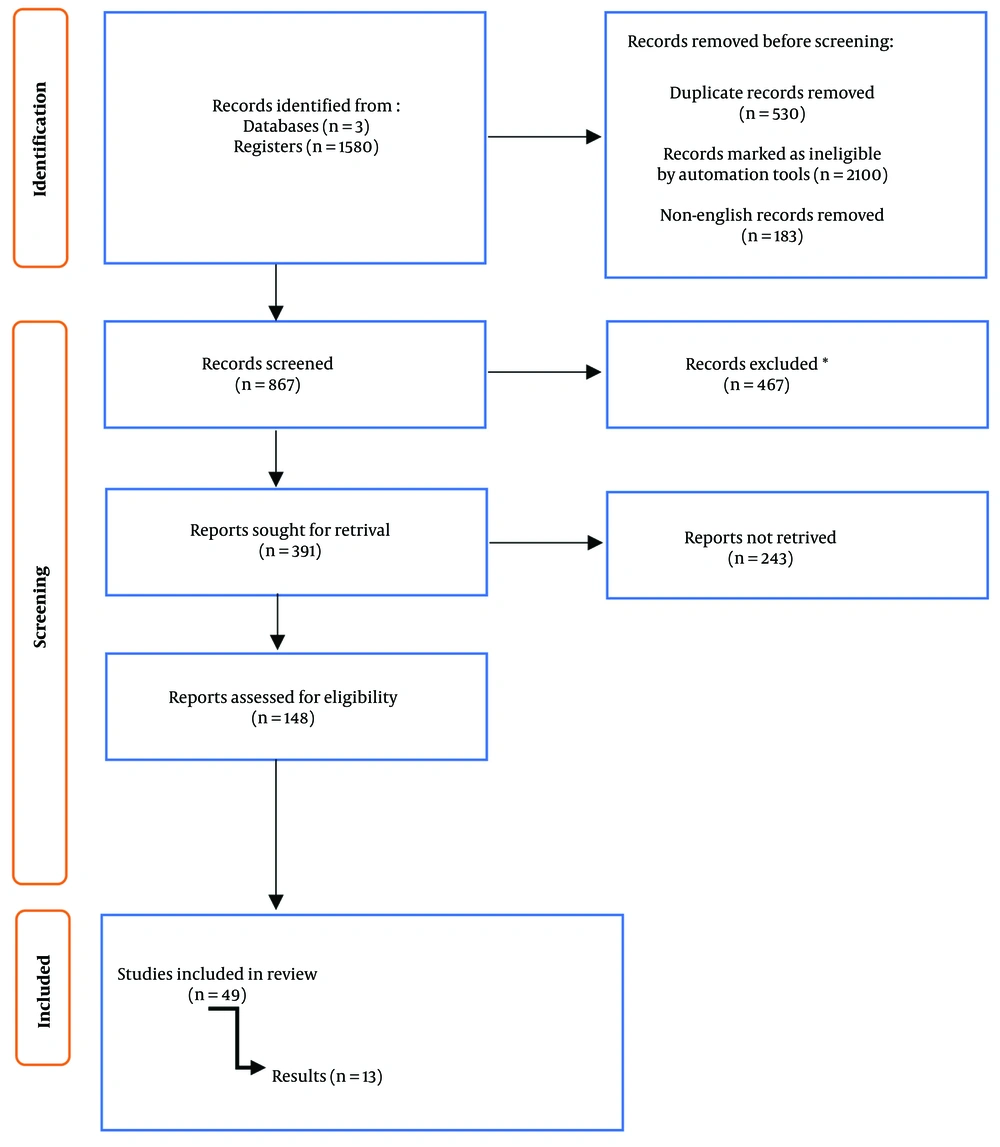
After excluding review articles, case reports, and letters to the editor retrieved from PubMed, Scopus, and Web of Science, duplicate entries were removed, and the remaining abstracts were screened. Full-text non-English papers were excluded after extensive screening. Forty-nine studies were screened independently by two teams of authors (Figure 2). The lead author and co-author, coordinating the process, performed an exhaustive screening, and thereby 13 articles pertaining to our research question were incorporated. Following the systematic review, we cross-checked the Cochrane library database for duplication and to ensure uniqueness. There were no duplicate or overlapping studies with similar results found.
4.2. Renal Cell Carcinoma Follows a Specific Janus Kinase/Signal Transducer and Activator of Transcription Signaling Pathway
All the articles reviewed discussed STAT pathways and kinases, with STAT3 being the most frequently reported signaling molecule in human renal carcinoma tissue cultures. Following STAT3, STAT1 was the most commonly mentioned growth factor in the literature. The measurement methods employed in these studies adhered to accepted standards, primarily utilizing Western blot and ELISA techniques to assess high-signaling pathways. Certain studies, such as those by Meng et al. and Deng et al., investigated tissue progression using STAT and JAK inhibitors, focusing on the STAT2, STAT3, and JAK2 pathways (17, 18). Fasler-Kan et al. identified STAT1, STAT3, and STAT6 pathways as phosphorylated types associated with interferon-γ (IFN-γ) and IFN-α (19). Tumors appear to exhibit elevated levels of STAT3, with Meng et al. also noting an increase in STAT2 within the same pathway. In RCC tissue, the STAT3 pathway is mediated by JAK2, while increased levels of IFN-γ and STAT1-JAK2, induced by INF, can be detected in RCC under high-chemokine conditions. Huang et al. proposed this pathway as a therapeutic target for RCC, demonstrating its inhibition using cinnamaldehyde. A group of genes associated with JAK/STAT signaling were found to be altered in renal tumors. Significant changes in STAT2 mRNA were detected using TIMER data, and the upregulation of IFN signaling genes, along with mediators such as JAK2, STAT1, and interferon-stimulated gene 15 (ISG15), as well as antiviral genes, was observed in renal cancer tissue. Collectively, the reviewed articles highlight the STAT pathway (particularly STAT3) and kinases, especially JAK2, as central signaling pathways in renal tumor progression. These findings clearly identify this signaling pathway as a critical factor for the diagnosis, prevention, and treatment of RCC (19-29).
4.3. Janus Kinase/Signal Transducer and Activator of Transcription Inhibitors Upregulate the Immune System to Reduce Renal Degeneration
Limiting the expression of SOCS genes presents a safe approach for treating and controlling RCC through the JAK/STAT signaling pathway. Several drugs have been shown to regulate STAT-related pathways, with cinnamaldehyde effectively suppressing the JAK2-STAT1/STAT3 pathway while increasing SOCS3 levels. Li et al.’s study demonstrated that the expression levels of these genes in examined tissues were associated with a reduction in their activity (26). In this study, SOCS3 and SOCS1 were identified as critical genes linked to the JAK/STAT signaling pathway in RCC. Huang et al. attributed the development of renal tumors and RCC pathology to the diminished inhibition of JAK/STAT by SOCS4 (24). While SOCS proteins are not the sole therapeutic targets, a group of proteins associated with this signaling pathway can be targeted to prevent the activation of necrotic and proinflammatory factors during cancer progression. Research has identified a broad spectrum of compounds capable of inhibiting tumor progression, including JAK/STAT antagonists. Guo et al. explored various compounds, with wogonin being the most significant, followed by baicalein and acacetin (27). Meng et al. utilized ruxolitinib to study the effects of GBP2 gene on STAT2 and STAT3, while AG490 was specifically linked to the kinase pathway (17). Figure 3 illustrates the most important compounds that can be employed to inhibit the JAK/STAT pathway in RCC.
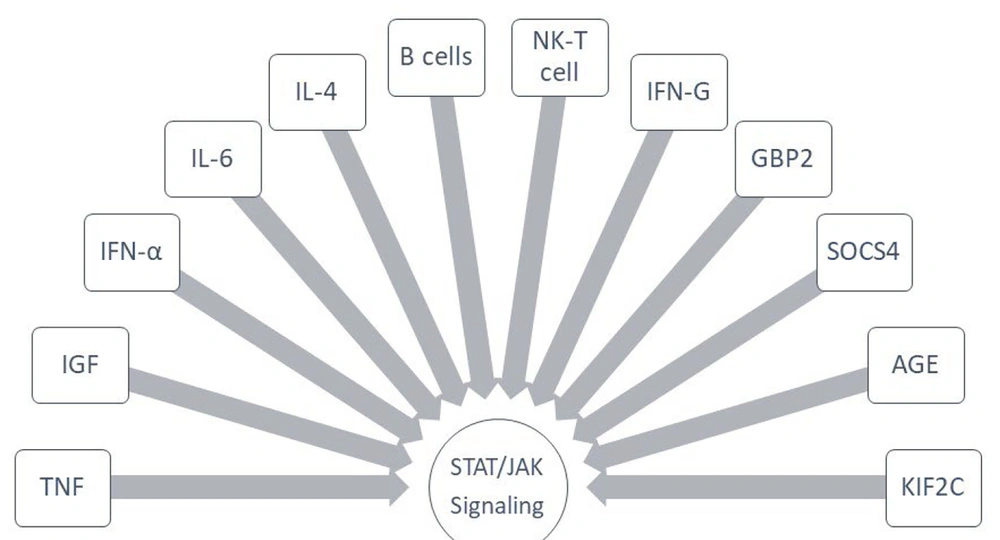
The most complexes that signaled Janus kinase/signal transducer and activator of transcription (JAK/STAT) in renal cell carcinoma (RCC). Abbreviations: IFN, interferon; TNF, tumor necrose factor; IGF, insulin-like growth factor; IL, interleukin; NK, natural killer; AGE, advanced glycation end products; KIF2C, kinesin family member 2C; SOCS, suppressors of cytokine signaling.
4.4. Janus Kinase/Signal Transducer and Activator of Transcription Protects Tumors from Immune System Poisoning
The JAK/STAT pathway, one of the most critical signaling pathways in the immune system, demonstrates a strong correlation with cytokines and inflammatory factors in RCC (Figure 4). A significant positive correlation was observed between STAT2 and T cells (CD3D, CD3E, and CD2) as well as B cells (CD19 and CD79A). Additionally, macrophages, neutrophils, and dendritic cells exhibit positive changes in response to STAT2 alterations. Available studies consistently highlight the upregulation of IFN and the JAK/STAT pathway. Liang et al. reported that this correlation extends to specialized subpopulations, including M2 macrophages, T Helper 1 (Th1) cells, T-bet cells, GATA3 cells, and Th2 cells (23). Furthermore, STAT3 shows a strong correlation with IL-12 and IL-15, while SOCS4 inhibits this pathway, playing a potential role in RCC treatment. The SOCS family is also linked to STAT6. Notably, STAT3, STAT6, and IRF1 are key signaling pathways within this family, with connections to growth factors such as vascular endothelial growth factor (VEGF). STAT3 targets genes associated with tissue inhibitors of metalloproteinases (TIMP1), which play crucial roles in tissue necrosis and fibrosis. Inhibitor complexes exhibit high affinity for STAT3, often maintaining this affinity with VEGF-A and epidermal growth factor receptor (EGFR). Reducing angiogenesis by limiting VEGF is considered a viable therapeutic strategy for RCC (30, 31). The JAK/STAT pathway facilitates bidirectional communication between proinflammatory factors, cytokines, and growth factors. Stimulation by TNF or IGF correlates with increased STAT1 activation, while IFN-α or IL-6 enhances STAT3, and IL-4 activates STAT6. In RCC, STAT2 expression is reduced in B cells, mesenchymal stem cells (MSCs), and NK T cells. IFN increases phosphorylated STAT1 levels, and IFN-γ enhances the JAK2/STAT1 pathway. JAK3, on the other hand, can be utilized to deplete Treg cells and modulate PD-1, cytotoxic T-lymphocyte associated protein 4 (CTLA4), lymphocyte-activation gene 3 (LAG3), T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), and granzyme B (GZMB). In conclusion, the relationship between the JAK/STAT pathway and inflammatory, necrotic, and angiogenic factors holds significant therapeutic potential for RCC. Suppressors of TNF, IFN, and IGF regulate the JAK/STAT pathway, leading to the cascade control of T and B cells, as well as necrotic positive regulators such as TIMPs.
5. Discussion
Focusing on signaling pathways as alternative treatments for inflammation and cancer offers a more accessible, tolerable, and patient-friendly approach. By concentrating on the components of the immune system, the balance of cytokines, and immune mediators, treatment strategies can be tailored to regulate inflammatory and necrotic factors in cancer. Chen et al. reported that propofol inhibits the progression of kidney cancer cells by modulating and reducing the expression of transforming growth factor beta 1 (TGF-β1), IL-6, tumor necrosis factor alpha (TNF-α), and hypoxia-inducible factor 1-alpha (HIF-1α), which are substrates of the sirtuin 1 signaling pathway (32). Our findings indicate that JAK3 activity extends to a broad range of T and B cells (33). FOXP3 facilitates the immune escape of kidney cancer cells, thereby limiting the ability to prevent metastasis. JAK3 signaling activates FOXP3 and other Tregs, suggesting its therapeutic potential (34, 35). Cancer cells often evade the toxicity of NK cells, and under complex conditions, the levels of these cells decrease. The STAT2 signaling pathway weakens NK cells, B lymphocytes, and mesenchymal cells (36, 37). Yin et al. using the HOOK1 complex, demonstrated increased NK cell activity and reduced angiogenesis, thereby preventing RCC metastasis (31). Vascular endothelial growth factor, a key angiogenesis-stimulating factor, plays a significant role in fetal development but also contributes to tumor metastasis, angiogenesis, and growth throughout the body (38). Recent studies have identified VEGF as a critical indicator of RCC (39, 40), making it a focal point in kidney immunotherapy. Guo et al.’s compounds inhibit the STAT3 pathway in association with vascular endothelial growth factor A (VEGFA) and EGFR, a mechanism similar to that of HOOK1 in NK cells (27).
While many immunotherapy drugs target the body’s defense mechanisms against tumors, controlling lymphocytes and fibrosis-promoting factors, our results emphasize a unified concept: Focusing on JAK/STAT inhibition enables the regulation of other signaling pathways in RCC. Inhibiting this pathway controls sub-signaling pathways involved in tumor growth, suppresses the expression of tumor growth factors such as TGF and TNF, and exposes cancer cells to effective immune system attacks. Although immunotherapy complexes in RCC impact the JAK/STAT pathway, certain drugs are specifically designed to target this pathway. Baricitinib, a selective JAK1/2 inhibitor, exhibits moderate effects on TYK2 with minimal inhibition of JAK3 (41). Tofacitinib, another selective JAK1 inhibitor, is used for rheumatoid arthritis (RA) patients with cardiovascular disease (42). Tofacitinib inhibits JAK/STAT, especially STAT3 and STAT1, and ruxolitinib inhibits the inflammatory pathway in this disease by suppressing the phosphorylation of STAT1 and STAT3 (43). EK100, an anti-inflammatory drug for immune-related diseases, was shown in our study to inhibit the JAK1/2 pathway, thereby controlling inflammation through the mitogen-activated protein kinase/activator protein-1 (MAPK/AP-1) pathway (44). In another model, flonoltinib maleate (FM) demonstrated greater inhibition of JAK2 compared to JAK1 and JAK3 (45). Guo et al. identified a range of protein and chemical complexes targeting this pathway, with wogonin being the most notable, alongside its control of STAT3, VEGFA, and EGFR (27). Deng et al. inhibited the JAK2/STAT3 pathway using AG490, a specific JAK inhibitor, highlighting the therapeutic potential of this signaling pathway (18).
Partial nephrectomy in the early stages of RCC is a good choice to control extensive metastasis, which is usually combined with other immunotherapies to form a multi-therapy treatment (44). In more severe cases, extensive nephrectomy is performed to remove the entire kidney and surrounding affected tissues and is supported by immunosuppressive therapies (45). In addition to losing part or all of the kidney, immunosuppressive therapies are associated with a high risk of other infections (46, 47). These types of therapies are associated with infectious and inflammatory problems because they do not specialize in each component of the immune system. What we have discussed as a therapeutic approach in our results is a new direction for pre-nephrectomy chemotherapy. Inhibition of STATs, in addition to preventing the progression of cancer tissue, allows tissue regeneration. The JAK/STAT inhibitors control parallel genetic pathways such as TIM-3 and CTLA4, which play a fundamental role in limiting cancer tissue. Overall, complexes acting as JAK/STAT antagonists are expected to control the progression of kidney tumors. Our findings indicate that this pathway serves both diagnostic and prognostic roles in RCC. By linking this signaling pathway to TGF-β, IFN, IL-6, and TNF, it reduces the expression of tumor growth factors. Suppressing this pathway enhances immune response functionality, reduces immune escape, and mitigates the loss of toxicity caused by NK cells in the tumor microenvironment of the kidney. Tumor metastasis to other organs, such as the bladder and upper organs like the lungs, can be effectively controlled using STAT3 inhibitors. Renal carcinoma represents a complex condition, and our discussion highlights the overarching role of JAK/STAT signaling. This systematic study presents unbiased results to guide future research into this pathway in other renal disorders. One of our studies revealed that high expression of STAT2 is associated with reduced mesenchymal activity in tuberculosis. This emerging topic has implications for both kidney transplantation and renal stem cell transplantation, warranting further detailed investigation.
5.1. Conclusions
The JAK/STAT signaling pathway interferes with RCC and can be used to disrupt tumor metastasis and prevent cell death. The inhibition of JAK/STAT signaling appears to be a specialized pathway for the treatment of renal carcinoma, with greater therapeutic effects and lower morbidity.
5.2. Limitation
Unfortunately, no human study using STAT and JAK inhibitors was available to us. A limitation of this treatment approach is the lack of human treatments, and the focus so far has been entirely on STATs in the form of in vitro models.
5.3. Future Research Directions
We strongly recommend that future studies use STAT inhibitors in the clinic to treat RCC to complete the results. It also seems that this pathway could be effective in nephrotic syndrome, which we suggest that further clinical studies be conducted on it.