1. Background
Hydrogen sulfide (H2S) is widely acknowledged for its significant physiological and pathological roles within the central nervous system. Functioning as a neuromodulator, H2S modulates neuronal and glial activity, facilitating long-term potentiation (LTP) and augmenting hippocampal N-methyl-D-aspartate (NMDA) receptor function — a region critical for learning and memory (1). Beyond neuromodulation, H2S demonstrates anti-inflammatory, antioxidant, and anti-apoptotic effects, which are implicated in alleviating central nervous system degenerative disorders, e.g., Alzheimer’s disease (AD) (2, 3). In AD contexts, H2S acts as a scavenger of reactive oxygen species (ROS), shielding neurons from oxidative stress, a key feature of neurodegeneration (4). Furthermore, H2S is integral to cognitive regulation, with studies highlighting its contribution to memory consolidation and learning processes (5). Both clinical and preclinical investigations consistently report diminished H2S levels in the plasma and brain tissues of individuals with cognitive disorders, including AD and schizophrenia, with these reductions correlating to the extent of memory impairment (6, 7). For example, in schizophrenia, lower H2S concentrations are linked to cognitive deterioration, emphasizing its potential role in cognitive dysfunction (8). Preclinical AD models reveal that depleted H2S exacerbates pathological progression, whereas exogenous H2S administration rescues cognitive deficits (9). Notably, intracerebroventricular (i.c.v.) formaldehyde administration in rats suppresses hippocampal H2S synthesis by downregulating cystathionine β-synthase (CBS), resulting in learning and memory impairments (10).
Conversely, sodium hydrosulfide (NaHS), an H2S donor, mitigates neuroinflammation and spatial memory deficits in β-amyloid (Aβ)-induced AD rat models (6). The NaHS also reduces Aβ (1 - 40)-triggered apoptosis in the hippocampal CA1 region and neutralizes oxidative stress-induced cytotoxicity (11, 12). In APP/PS1 transgenic mice, H2S supplementation enhances spatial memory, curtails Aβ generation, and diminishes senile plaque deposition, likely through oxidative stress inhibition and antioxidant pathway activation (13). However, the therapeutic potential of NaHS in the streptozotocin (STZ)-induced rat model of AD remains unexplored. The present study aims to investigate this potential.
2. Objectives
To determine the protective effects of exogenous H2S, administered as NaHS, on STZ-induced impairments in learning and memory retrieval in rats.
3. Methods
3.1. Animals
Sixty adult male Wistar rats (180 - 200 g) were housed in standard polycarbonate cages under controlled environmental conditions (temperature: 23 ± 3°C; 12-h light/dark cycle). Animals had free access to standard rodent chow and water. Behavioral experiments were conducted during the light phase (08:00 - 16:00).
3.2. Experimental Design
To evaluate the protective effects of NaHS, an H2S donor, on STZ-induced memory impairment, rats were randomly allocated to five groups (n = 10): (1) Control (intact), (2) sham (vehicle injection), (3) STZ, (4) STZ + vehicle (saline), and (5) STZ + NaHS (5.6 mg/kg, Sigma-Aldrich, USA). Rats in the NaHS group received daily intraperitoneal (i.p.) injections of NaHS (5.6 mg/kg, Sigma-Aldrich, USA) dissolved in normal saline for 21 days post-surgery. Learning and memory were assessed using the passive avoidance (PA) test on day 22 following STZ administration.
3.3. Induction of Alzheimer’s-Like Pathology
Intracerebroventricular (i.c.v.) The STZ administration was used to model sporadic AD pathology by inducing brain insulin resistance, as previously described by Sharma and Gupta. Ketamine hydrochloride (60 mg/kg, i.p.; Alfasan) and xylazine (8 mg/kg, i.p.; Alfasan) were injected, and then the rats were secured in a stereotaxic apparatus (Stoelting, USA). The STZ (3 mg/kg, 5 µL per ventricle; Sigma-Aldrich) was dissolved in distilled water and administered bilaterally into the lateral ventricles (coordinates: AP: -1.0 mm, ML: ±1.4 mm, DV: -3.4 mm relative to bregma) using a Hamilton syringe. Sham and vehicle groups received equivalent volumes of distilled water or saline, respectively (14).
3.4. Passive Avoidance Test
This device was employed to assess learning and memory performance using a two-chamber shuttle box (20 × 30 × 20 cm) consisting of a light and a dark compartment divided by a guillotine door (6 × 8 cm). The dark compartment’s floor was equipped with stainless steel rods (1 cm apart) connected to a shock generator (Borj Sanat, Iran). The test comprised three phases: Habituation, acquisition, and retention.
For habituation, rats were placed in the light compartment and allowed to explore both compartments for 5 minutes with the guillotine door open. In the acquisition phase, following habituation, rats underwent three 5-minute trials (30-minute inter-trial interval). Upon entering the dark compartment during the third trial, the door was closed, and a single foot shock (1.5 mA, 50 Hz, 1 s) was delivered via the grid floor. Rats remained in the dark compartment for an additional 10 seconds to reinforce the association between the dark compartment and the shock. This procedure was repeated after 2 minutes if the rat re-entered the dark part of the apparatus. Acquisition was considered complete if the rat avoided the dark compartment for 120 seconds.
To evaluate retention, forty-eight hours post-acquisition, memory retention was tested. Rats were positioned in the light compartment, and the step-through latency (STL, time to enter the dark compartment), total time spent in the dark compartment (TDC), total time spent in the light compartment (TLC), and the number of entries into the dark compartment were recorded over 600 seconds. No shock was delivered during this phase. Impaired memory retention was indicated by reduced STL, increased TDC, and a higher number of dark compartment entries.
3.5. Statistical Analysis
All data are presented as mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test. The collected data were analyzed using SPSS software v: 29.0.2.0. A P-value of less than 0.05 was considered statistically significant.
3.6. Ethical Considerations
The principles of medical research ethics were followed in accordance with the Declaration of Helsinki and the guidelines of the National Committee on Ethics in Medical Research. The proposal was approved by the Medical Ethics Committee of Qazvin University of Medical Sciences (IR.QUMS.REC.1396.106). Anesthesia was administered during surgical procedures to ensure animal welfare.
4. Results
4.1. Effect of Streptozotocin on Passive Avoidance Memory Performance
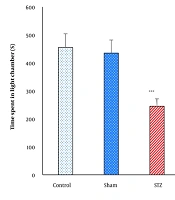
The STL, TLC, TDC, or the number of entries into the dark compartment during the retention test did not show significant differences between the control and sham groups (P > 0.05, Figures 1 - 4). In contrast, the STZ group exhibited significant impairments in memory performance. Specifically, STL and TLC were significantly reduced (P < 0.001), while TDC and the number of dark compartment entries were significantly increased (P < 0.001) compared to the control and sham groups, indicating STZ-induced memory deficits (Figures 1 - 4).
4.2. Effect of Sodium Hydrosulfide Treatment on Streptozotocin-Induced Memory Impairment
Treatment with NaHS (5.6 mg/kg, i.p.) significantly ameliorated STZ-induced memory impairments in the PA test. Compared to the STZ and STZ + Saline groups, the STZ + NaHS group showed a significant increase in STL (P < 0.05, Figure 1) and TLC (P < 0.05, Figure 2), alongside a significant decrease in TDC (P < 0.05, Figure 3) and the number of dark compartment entries (P < 0.05, Figure 4). These findings suggest that NaHS treatment effectively mitigated STZ-induced memory deficits.
5. Discussion
The present study investigated the neuroprotective potential of NaHS, a H2S donor, against an intracerebroventricular STZ-induced rat model of AD. Our findings demonstrate that NaHS treatment for 21 days significantly attenuated STZ-induced cognitive impairment, as evidenced by improved performance in PA tests. Specifically, NaHS reversed STZ-induced decreases in STL and time spent in the light chamber, while reducing time spent in the dark chamber and entries into the dark part of the shuttle box. These results suggest that H2S ameliorates synaptic dysfunction and memory decline associated with STZ administration, highlighting its therapeutic potential in AD-like pathology.
The mechanism of the positive effect of H2S on improving cognitive disorders in AD has not yet been fully determined, but based on previous studies, several reasons can be cited for its protective effects, such as its antioxidant, anti-inflammatory, and anti-apoptotic properties (15). The neuroprotective properties of H2S identified in this study may be attributed to its well-documented functions in regulating oxidative stress and inflammatory responses. As an effective neutralizer of ROS, H2S bolsters endogenous antioxidant mechanisms, such as glutathione production and superoxide dismutase activity (16).
Supporting these observations, prior research has illustrated the neuroprotective efficacy of NaHS across diverse pathological contexts (17). For example, another study found that NaHS alleviates homocysteine (Hcy)-induced cognitive impairment by diminishing reactive aldehyde buildup via enhanced glutathione levels and aldehyde dehydrogenase 2 (ALDH2) activation (10). Parallel findings by Karimi et al. revealed that NaHS mitigates cognitive deficits following traumatic brain injury (TBI) (18). Further studies indicate that NaHS reduces Aβ25 - 35-triggered neuronal degeneration, inflammatory markers, and apoptotic pathways (6, 19). Zhang and Bian demonstrated that NaHS decreases apoptosis and downregulates autophagy-related proteins, such as Vps34, Beclin-1, and LC3II, in brain-injured models (20). Additionally, Jiang et al. linked NaHS to attenuated TBI-induced blood-brain barrier (BBB) dysfunction, cerebral edema, and lesion size, correlating these effects with reduced oxidative stress and elevated antioxidant enzyme activity (21). In cerebral ischemia, NaHS was shown to dose-dependently diminish brain injury and post-ischemic edema (22, 23). Notably, H2S, a metabolite of NaHS, acts as a ROS scavenger, protecting neurons from oxidative damage (24), which suggests antioxidant mechanisms underpin its neuroprotective role. Lu et al. further reported that H2S enhances astrocytic glutamate uptake under H2O2-induced stress, underscoring its therapeutic relevance in oxidative brain injury (25). The NaHS also promotes hippocampal neuron survival during oxygen-glucose deprivation and reoxygenation (26).
Beyond antioxidant effects, Lu et al. identified H2S as a regulator of intracellular pH in neural cells (27), while Gong et al. observed that NaHS counteracts lipopolysaccharide (LPS)-induced cognitive decline by suppressing pro-inflammatory mediators (28). Chu et al. extended these findings, showing preoperative NaHS administration alleviates surgery-related memory deficits via reduced systemic and cerebral pro-inflammatory cytokine levels (29). Collectively, these studies emphasize the dual antioxidant and anti-inflammatory pathways through which NaHS exerts its protective neural effects, offering mechanistic insights into its therapeutic potential.
5.1. Conclusions
The present study demonstrates that systemic NaHS administration improves cognitive deficits induced by STZ. Our findings provide novel evidence for the protective role of NaHS against STZ-induced cognitive impairment. Based on existing literature and current results, NaHS reduces glutamate excitotoxicity, oxidative stress, and enhances antioxidant enzyme activity in the brain, and may serve as a promising neuroprotective agent for neurodegenerative disorders characterized by intellectual decline, such as AD.
![Step-through latency (STL); comparison of STL during the retention test across experimental groups [***P < 0.001 vs. control and sham groups; +P < 0.05 vs. streptozotocin (STZ) and STZ + saline groups]. Step-through latency (STL); comparison of STL during the retention test across experimental groups [***P < 0.001 vs. control and sham groups; +P < 0.05 vs. streptozotocin (STZ) and STZ + saline groups].](https://services.brieflands.com/cdn/serve/3170c/720c45c56128c4067c93588f8014fff4d0099114/jid-29-3-162588-i001-preview.webp)
![Total time in light compartment (TLC); comparison of TLC during the retention test across experimental groups [***P < 0.001 vs. control and sham groups; +P < 0.05 vs. streptozotocin (STZ) and STZ + saline groups]. Total time in light compartment (TLC); comparison of TLC during the retention test across experimental groups [***P < 0.001 vs. control and sham groups; +P < 0.05 vs. streptozotocin (STZ) and STZ + saline groups].](https://services.brieflands.com/cdn/serve/3170c/257a9c8975fdf287dd478dbed9ab96e21b38a708/jid-29-3-162588-i002-preview.webp)
![Total time in dark compartment (TDC); comparison of TDC during the retention test across experimental groups [***P < 0.001 vs. control and sham groups; +P < 0.05 vs. streptozotocin (STZ) and STZ + saline groups]. Total time in dark compartment (TDC); comparison of TDC during the retention test across experimental groups [***P < 0.001 vs. control and sham groups; +P < 0.05 vs. streptozotocin (STZ) and STZ + saline groups].](https://services.brieflands.com/cdn/serve/3170c/4c2468d203068bb56d16582aed673f5f2fbf910c/jid-29-3-162588-i003-preview.webp)
![Number of dark compartment entries; comparison of the number of entries into the dark compartment during the retention test across experimental groups [***P < 0.001 vs. control and sham groups; +P < 0.05 vs. streptozotocin (STZ) and STZ + saline group]. Number of dark compartment entries; comparison of the number of entries into the dark compartment during the retention test across experimental groups [***P < 0.001 vs. control and sham groups; +P < 0.05 vs. streptozotocin (STZ) and STZ + saline group].](https://services.brieflands.com/cdn/serve/3170c/d0964abb570683ef29582030d6df7a7163ef0319/jid-29-3-162588-i004-preview.webp)