1. Context
Kidney disease is a severe problem worldwide, and its prevalence has been increasing in recent years. Kidney disease is divided into two main categories: Acute kidney injury (AKI) and chronic kidney disease (CKD) (1). Acute kidney injury is a condition in which kidney function declines abruptly, lasting for seven to 90 days, resulting in decreased glomerular filtration rate and urine output, as well as increased serum creatinine (2, 3). Chronic kidney disease is a broad term that refers to a group of diseases that impair the structure and function of the kidneys for more than three months and progress to end-stage renal disease (ESRD) (4, 5). Disease expression variation is influenced by the etiology, pathophysiology, severity, and progression rate (6).
Kidney transplantation and dialysis are the two main treatments currently available for kidney regeneration. Both are unsatisfactory, and the increase in survival rates after treatment is insufficient. Dialysis has several drawbacks, including high mortality rate, hospitalization, loss of independence, depression, and high drug costs (7). The limitations of kidney transplantation are the shortage of kidney donors, the risk of infection or cancer transmission, the high cost of the surgeon, and severe immune rejection (8). As a result, the development of practical therapeutic approaches has opened up new opportunities for kidney regeneration (9, 10).
Over the last few decades, many studies have shown that transplanting stem cells to patients with kidney disease improves their kidney function (11). Among all stem cell types, mesenchymal stem cells (MSCs) have been identified as one of the most effective cell types for inducing kidney regeneration due to their ease of isolation and expansion and lack of teratoma risk, immunosuppressive properties, and absence of ethical problems (12, 13). Despite the benefits of MSC-based therapy, there are some drawbacks, including the possibility of tumorigenesis, prion, and viral transmission, loss of differentiation and morphological changes after long-term culture, and the possibility of antibody production in the host body after repeated administration of MSCs (14, 15). Cell-free approaches have been used in recent years to reduce the adverse effects of MSC-based cell therapy. Exosomes are a group of extracellular vesicles with sizes ranging from 30 to 100 nm produced inside multivesicular bodies (MVBs) in almost all types of cells and secreted from the original cell to the target recipient cell, which is one of the tools used in cell-free approaches (16, 17). As known, MSC-exosomes contain pro-regenerative molecules from their origin cell and mimic MSC functions in tissue regeneration in cell therapy, mediating intercellular communication; thus, they are a popular substitute for cell therapy and have the potential to regenerate tissues and be used in tissue engineering without the risk of tumorigenesis or high immune rejection (18, 19).
Aside from the availability of diagnostic markers for AKI and CKD, such as serum creatinine and urine output, urinary exosomes have aided in making the diagnostic process more sensitive and faster. Changes in the expression of specific molecules in urinary exosomes derived from the kidney, prostate, and bladder organs can be used as biomarkers to assess kidney health. The lipid bilayer structure of exosomes protects their cargo from degradation, allowing them to be isolated and analyzed for differences in disease biomarker expression levels (20). For example, in ischemia/reperfusion (I/R) models, miRNAs of urinary exosomes revealed the state of kidney injury or fibrosis (21).
This review article will concentrate on the role of MSC-derived exosomes in treating kidney disease and summarize the latest findings on the diagnosis and application of urinary exosomes.
2. Evidence Acquisition
2.1. Acute Kidney Injury
Acute kidney injury is a sudden impairment of renal function and structure associated with a high morbidity and mortality rate in hospitalized patients. Clinical signs of AKI include a sudden rise in serum creatinine, a decrease in urine volume, and a fall in glomerular filtration rate (GFR) (2, 3, 22). The most important definitions of AKI are based on RIFLE (risk, injury, failure, loss, and end-stage renal disease) classification, AKIN (AKI network), and KDIGO (kidney disease improving global outcomes) (Table 1) (2, 23, 24).
| Class | RIFLE SCr or GFR | Stage | AKINSCr | Stage | KDIGO SCr |
|---|---|---|---|---|---|
| Risk | Increased SCr × 1.5 or GFR decrease > 25% (within 7 days) | 1 | Increase in SCr ≥ 0.3 mg/dL or ≥ 150% to 200% (1.5 to 2-fold) from baseline (within 48 hours) | 1 | Increase in SCr by ≥ 0.3 mg/dL within 48 hours or increase in SCr 1.5 to 1.9 times the baseline which is known or presumed to have occurred within the prior 7 days |
| Injury | Increased SCr × 2.0 or GFR decrease > 50% | 2 | Increase in SCr to more than 200% to 300% (> 2 to 3-fold) from baseline | 2 | Increase in SCr to 2.0 to 2.9 times the baseline |
| Failure | Increased Scr × 3.0 or GFR decrease > 75% or SCr ≥ 4.0mg/dL or acute increase ≥ 0.5 mg/dL | 3 | Increase in SCr to more than 300% (> 3-fold) from baseline or SCr ≥ 4.0 mg/dL with an acute increase of at least 0.5 mg/dL or initiation of renal replacement therapy | 3 | Increase in SCr to 3.0 times the baseline increase in SCr to ≥ 4.0 mg/dL or initiation of renal replacement therapy |
| Loss | Persistent acute renal failure = Complete loss of kidney function > 4 weeks | ||||
| End-stage kidney disease | End-stage of kidney disease (> 3 months) |
Acute Kidney Injury Definition Based on RLIFE, AKIN, and KDIGO
The area, number of patients, and definition of AKI play a role in AKI epidemiology (25, 26). In affluent countries, hospital-acquired AKI is more common in older and severely ill patients (27-29). The leading causes of AKI in developing countries, depending on patient accommodation, are healthcare-related conditions such as nephrotoxic drugs and sepsis in urban areas and community-acquired conditions such as infectious disease and diarrhea in rural areas (30-33). A meta-analysis of 154 studies based on the KDIGO definition of AKI was conducted to estimate the global incidence of AKI in developed countries in North America, Northern Europe, and Eastern Asia. They discovered that one in every five adults and one in every three children in the world suffer from AKI during a hospital stay (34). The etiology of AKI is divided into three categories: Pre-renal, post-renal, and intrinsic (35, 36). Glomerular filtration rate decreases in the pre-renal category without impairment of the renal parenchyma; the leading causes in this category are renal hypoperfusion, cardiac failure, intravascular depletion, sepsis, hypotension, pancreatitis and liver diseases (cirrhosis), bleeding, and burns (37-39). Acute obstruction of the urine flow, such as ureteric calculus, causes post-renal AKI, which causes an increase in intra-tubular pressure, impairment of renal blood flow, and inflammations lowering GFR, eventually leading to renal failure (40, 41). Intrinsic AKI, also known as acute renal failure, is when the kidney suffers from various direct and sudden damages. The most common causes of intrinsic AKI are Acute Tubular Necrosis (ATN), nephrotoxins, vasculitis, bacterial or viral infections, allergic interstitial nephritis, hepatorenal syndrome, and glomerulonephritis (2, 36, 42).
2.2. Chronic Kidney Disease
Chronic kidney disease is defined as persistent abnormalities in the structure and function of the kidney lasting for more than three months or a reduction in GFR of less than 60 mL/min per 1.73 m2 (4). The CKD global incidence is increasing, with a global prevalence of 13.4%, and the number of ESRD people ranges from 4.902 to 7.083 million (43). The CKD etiologies include AKI, hypertension, toxic insults, diabetes mellitus, age, obesity, and nephrectomy (6, 44). Besides, CKD has a wide range of characteristics and mechanisms, some of which are associated with AKI pathologic mechanisms. Also, CKD is characterized by pericyte migration, pericyte phenotype change, microvascular loss, chronic tubular hypoxia, cellular senescence, collagen deposition, myofibroblast proliferation, tubular loss, and replacement with collagen scars, chronic leukocyte infiltration, and end-stage renal failure (45-48). End-stage renal failure (ESRD) is the final stage of CKD in which the kidney fails, and dialysis or kidney transplantation is required to survive (49). Diabetes mellitus (DM) is one of the major causes of CKD, in which the inflammation and oxidative stress of the kidney, resulting from hyperglycemia, lead to the production of inflammatory cytokines and the development of diabetic nephropathy. Diabetic nephropathy (DN) is a diabetic kidney disease that results in ESRD by causing structural (glomerular basement membrane thickening and glomerular sclerosis) and functional (GFR reduction, albuminuria, proteinuria, and hyperfiltration) changes in the kidney (50, 51). Because of the difficulty of diagnosing CKD in the early stages due to the asymptomatic condition, disease diagnosis is delayed, and available treatments for CKD regeneration are limited.
2.3. Exosomes
2.3.1. Discovery and Definition of Exosomes
For the first time, scientists observed 50 nm extracellular membrane vesicles carrying transferrin receptors releasing from reticulocytes in 1938. Multivesicular endosomes holding vesicles with transferrin receptors were observed fusing to the membrane of a sheep reticulocyte and releasing those vesicles into the extracellular environment; and in 1987, the term “exosome” was coined to describe this type of extracellular vesicle (52, 53). Exosomes are cup-shaped lipid bilayer vesicles with a size of 30 - 100 nm that are secreted by a variety of cells, including MSCs, neuronal cells, cytotoxic T cells, and platelets, and found in various body fluids, including sperm, blood, urine, and amniotic fluid (16, 54, 55).
2.3.2. Biogenesis of Exosomes
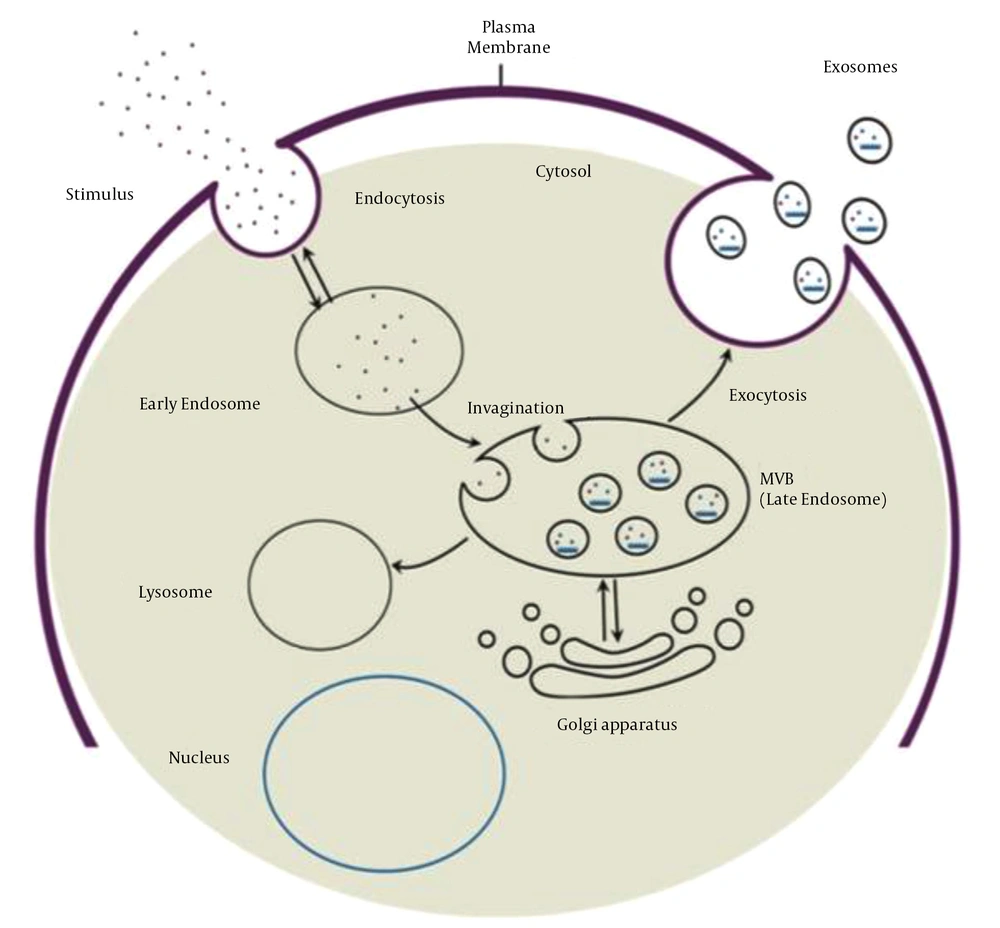
Exosome biogenesis begins with the maturation of an early endosome to a late endosome or MVB. Invagination of the endosomal membrane during late endosome formation results in the absorption of intracellular content and the formation of intraluminal vesicles. Multivesicular bodies have two fusion pathways: Fusion to the lysosome and degradation of the cargo, and fusion to the plasma membrane and release of intraluminal vesicles called exosomes into the extracellular space (Figure 1). Evidence suggests that two distinct mechanisms can form intraluminal vesicles: Endosomal sorting complex required for transport (ESCRT)-independent and ESCRT-dependent mechanisms. ESCRT is composed of four complexes and accessory proteins: ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III. These complexes cooperate in an orderly manner to identify ubiquitinated proteins in the endosomal membrane and form intraluminal vesicles via inward budding. The ESCRT-independent mechanism, on the other hand, is dependent on lipid raft microdomains enriched with sphingomyelinases and tetraspanin-enriched microdomains (56-58).
Exosome biogenesis process and two fusion pathways of MVB (59)
2.3.3. Components of Exosome
Exosomes are complex entities whose contents depend on the parent cell. Exosomes contain various elements, including lipids, proteins, and nucleic acids. Tetraspanins (CD63, CD81, CD82, and CD9) as surface markers, heat shock proteins (HSP70, HSP90), MVB formation and release proteins (ESCRT complex, Alix, and TSG101), and MVB membrane transport and fusion (GTPases and annexins, phospholipase and lipid-related proteins) are all common proteins found in exosomes. Exosomes contain a variety of RNA patterns, including lncRNA, tRNA, mRNA, miRNA, and rRNA, which are involved in several biological functions in the recipient cell. According to the data, microRNA is the most abundant RNA pattern in exosomes. Sphingomyelin, prostaglandins, cholesterol, phosphatidylserine, various fatty acids, and leukotrienes make up the lipid content of exosomes, which play significant roles in structural stiffness and the protection of the inner cargo of exosomes from degradation (58, 60-62).
2.3.4. Isolation of Exosomes
Exosome purification from bodily fluids and conditioned culture media necessitates tools and established procedures. There are several suggested approaches, each with its advantages and disadvantages. Differential ultracentrifugation is the most commonly used method due to advantages such as low cost, short processing time, and density-based exosome purification. However, the lack of specificity of this method is a significant drawback, as it prevents complete purification and permits contamination with other extracellular vesicles, protein aggregates, and genetic elements. Furthermore, due to the presence of complex elements in urine and serum, differential ultracentrifugation is ineffective for purifying their exosomes. Low yield and purification necessitate the use of differential ultracentrifugation in conjunction with a sucrose cushion to increase specificity (63-67). Exosomes are filtered from smaller contaminants using the ultrafiltration purification technique in a quick, easy, and high-yield resolution process; however, there is a risk of contamination fragmentation depending on the pore size. As a result, some particles and fragments of the same size as exosomes can pass through the pores using this mechanism (68, 69). Another method for exosome purification is high-pressure liquid chromatography (HPLC), which creates a homogeneous environment for exosomes while preserving their biological properties. Although this method is highly purifying, it is expensive and has limited scalability (70, 71). Immunoaffinity capture uses antibodies that bind to surface markers on exosomes; however, it produces highly purified exosomes, is expensive, and has a low yield (72). ExoQuick precipitation methods enable the production of highly purified exosomes from a small sample volume in less than two hours (73). Polymer-based precipitation, Microfluidic technology, field-flow fractionation, and a combination of these techniques are used for isolation; therefore, given the impossibility of removing all contaminants, selecting an appropriate approach to provide the most purified exosomes is critical (64, 74).
2.3.5. Identification of Exosomes
Following exosome isolation, it is critical to confirm their characteristics and features. Exosome surface markers are identified using immunocytochemical analysis, western blot, and flow cytometry (CD81, CD63, CD82, and CD9). Atomic force microscopy (AFM), Scanning Electron Microscopy (SEM), and transmission electron microscopy (TEM) can all be used to examine morphology and size (75, 76). Exosome concentration and size distribution can be determined using dynamic light scattering (DLS) and nanoparticle tracking analysis (NTA) (77-79). However, the quick and inexpensive ELISA method is the gold standard for quantifying exosomes and their markers (80). In recent years, surface-enhanced raman spectroscopy (SERS) has been introduced as a precise and sensitive method for identifying and analyzing exosome-like vesicles (81) (Table 2).
| Variables | Exosome Features |
|---|---|
| Diameter and shape | 30 - 100 nm, cup shape |
| Origin | Release of exosomes to extracellular matrix after fusion of multivesicular body with plasma membrane |
| Protein content | CD63, CD81, CD82, CD9, HSP70, HSP90, ESCRT complex, Alix, TSG101, GTPases, annexins, phospholipase, and lipid-related proteins |
| Lipid content | Phosphatidylserine, various fatty acids, leukotrienes sphingomyelin, prostaglandins, and cholesterol |
| Nucleic acids | miRNA, mRNA, DNA, and non-coding RNA |
| Isolation techniques | Ultracentrifugation, ultrafiltration, immunoaffinity capture, high-pressure liquid chromatography, and precipitation |
| Identification techniques | Immunocytochemical analysis, western blot, flow cytometry, microscopic analysis, ELISA, dynamic light scattering, nanoparticle tracking analysis, surface-enhanced Raman spectroscopy |
Summary of Exosome Features, Isolation, and Identification Methods
2.3.6. Function of Exosomes
Exosomes have a variety of functions depending on their origin cells. They participate in immune responses, inflammation, angiogenesis, coagulation, cell-to-cell communication, and spreading pathogens such as prions and viruses. They also play essential roles in disease diagnosis, cell-free therapy, and delivery (proteins, genes, and chemicals) (82-84). This review article concentrates on the diagnosis and regenerative applications of MSC-derived exosomes in kidney disease.
2.4. MSC-derived Exosomes
In 2010, Lai et al. found the paracrine influence of MSCs on tissue regeneration and demonstrated for the first-time particles with a size of 50 - 100 nm called exosomes secreting from MSCs in ischemic/reperfusion injury mice (85). As known, MSC-exosomes have biological functions similar to their origin cells, and depending on the content; they can repair cells and tissues, regulate immune and inflammatory responses, suppress apoptosis, and modulate homeostasis, cell growth, proliferation, survival, migration, tumor progression, and inhibition (86, 87).
There are numerous cargos in the structure of MSC-exosomes that differ depending on the source cell. Tetraspanins, adhesion proteins, antigen-presenting proteins, cytokine receptors, heat shock proteins, lipoproteins, fatty acid-binding proteins, trophic factors, cytokines, chemokines, membrane fusion proteins, translation and transcription proteins, motility proteins, structure proteins, and enzymes are among the nearly 2000 proteins identified in MSC-exosomes. The nucleic acid content of exosomes is enriched with miRNAs, which are involved in various regulatory and pathological states such as angiogenesis, inflammation, cell growth, tumorigenesis, and tumor progression. Besides, MSC-exosome lipid content includes various types of fatty acids, prostaglandins, lysophosphatidylcholine, leukotrienes, and other lipids found in all exosomes (88, 89).
The widespread availability of MSCs and large-scale production of exosomes for cell-free therapy are some of the benefits of using MSC exosomes as a therapeutic tool (90, 91). Also, MSC-exosome isolation is more straightforward, less time-consuming, and less expensive than MSC isolation (92). Furthermore, exosome therapy is safer than MSC-based therapies because exosomes lack the potential to multiply and are free of a substantial quantity of markers that can be shown as antigens by the host body, resulting in reduced immunological rejection (93). In addition, there are no concerns about cell survival in exosome-based regeneration, and structural stability for an extended time at a storage temperature of 20°C makes them an excellent choice for cell-free therapy (94) (Table 3).
| Kidney Disease | Role of Exosome | Main Results | Reference |
|---|---|---|---|
| AKI | Biomarker | Upregulation of miR-16, miR-24, miR-200c, miR-125, miR-351, miR-9a, miR-141, miR-200a, miR-200c, and miR-429 | (21) |
| Biomarker | Downregulation of AQP-2 and AQP-1 after 168 hrs, upregulation of AQP-1 after 24 hrs | (21, 95) | |
| Biomarker | Upregulation of uATF3 | (96) | |
| Biomarker | Upregulation of miR-30c-5p and miR-192-5p | (97) | |
| CKD | Biomarker | Upregulation of miR-21 and downregulation of miR‑29c | (98) |
| Biomarker | Downregulation of miR-29c-5p and miR-15b-5p, upregulation of let-7c-5p | (99) | |
| Biomarker | Upregulation of miR-30a, miR-133b, and miR-342 | (100) | |
| Biomarker | Upregulation of miR-21 | (101) | |
| Biomarker | Downregulation of miR-200b | (102) | |
| Biomarker | Upregulation of miR-451 | (103) | |
| AKI | Therapeutic | Proliferation of injured PTECs | (104) |
| Therapeutic | Enhancement of cell proliferation, anti-apoptotic and antioxidant effects | (105) | |
| Therapeutic | Reduction of Sema3A, cleaved caspase 3, and pro-apoptotic protein Bax., upregulation of Bcl-2 | (106) | |
| Therapeutic | Upregulation of autophagosome marker LC3B, reduction of inflammation and apoptosis, inhabitation of mTOR pathway | (107) | |
| Therapeutic | Downregulation of genes related to hypoxia, apoptosis, and cytoskeleton reorganization | (108) | |
| Therapeutic | Antioxidant effect by upregulation of HO-1 and Nrf2/anti-oxidant response element | (109) | |
| Therapeutic | Enhancement of autophagy and prevention of nephrotoxicity | (110) | |
| Therapeutic | Reduction of CCL2 concentration and recruitment of macrophages/monocytes for inflammation | (111) | |
| Therapeutic | Decreasing oxidative stress, inflammation, fibrotic, and apoptotic biomarkers, increasing anti-apoptotic and angiogenesis biomarkers | (112) | |
| CKD | Therapeutic | Decreasing pro-inflammatory cytokines, medullary oxygenation, and fibrosis | (113) |
| Therapeutic | Reducing fibrosis and downregulating fibrotic genes | (114) | |
| Therapeutic | Reduction of EndoMT and apoptosis, enhancement of endothelial cell proliferation, inhabitation of kidney fibrosis | (115) | |
| Therapeutic | Promoting angiogenesis, reducing microvascular architecture, and endothelial cell apoptosis | (116) | |
| Therapeutic | Reduction of renal ischemia, hypoxia, subsequent fibrosis, and infiltration of inflammatory cells | (117) | |
| Therapeutic | No regenerative effects | (118) | |
| Therapeutic | Suppressing renal fibrosis and EMT and protecting tubular endothelial cells | (119) | |
| Therapeutic | Downregulating of mTOR and fibrotic marker expression Improvement of renal function and morphology | (120) | |
| Therapeutic | Promoting vascular regeneration and cell survival, reduction of urine volume and albumin excretion, increasing glomerular endothelial cell proliferation | (121) |
Summary of Roles of Urinary Exosomes in Diagnosis and MSC-derived Exosomes in Regeneration of Acute Kidney Injury and Chronic Kidney Disease
2.5. Diagnosis Application of Urinary Exosomes in Renal Disease
The complex and unique structure of exosomes, which resembles the constitution of their parent cell, is a tool for demonstrating cellular mechanisms in the body and can thus be used as a biomarker for disease diagnosis. Recent research has shown that exosomes derived from luminal epithelial renal cells reflect renal function. Consequently, urinary exosomal biomarkers can be used to diagnose AKI and CKD.
2.5.1. AKI Diagnosis
Urine analysis, urine output measurement, and blood tests are standard AKI diagnostic tools. On the other hand, Urinary exosomes are valuable sources for diagnosing AKI in a precise and timely manner. According to one study, increased levels of urinary exosomes indicated the injury phase. In contrast, the upregulation of miR-9a, miR-141, miR-200a, miR-200c, and miR-429 indicated the early recovery phase and increased expression level of miR-125 and miR-351 indicated the late stage of the fibrotic phase of ischemia/reperfusion-induced AKI in rat models (21). In another study, researchers assessed the expression levels of urinary exosomal aquaporin-1 (AQP-1) and aquaporin-2 (AQP-2) to see if these assessments can be applied to detect early and late stages of cisplatin-induced AKI in rat models. They observed that a decrease in AQP-2 level of the inner medulla after 168 h, an increase in AQP-1 level of the outer medulla after 24 h, and a reduction after 168 h are the markers of renal impairment in rat models (21, 95). In sepsis-induced AKI mice models, the upregulation of urinary exosomes' activation transcriptional factor 3 (uATF3) was a biomarker for disease diagnosis (96). Besides, miR-30c-5p and miR-192-5p upregulations have been identified as promising biomarkers for ischemia/reperfusion-induced kidney injury (97). 2.5.2. CKD Diagnosis
Many researchers who have looked into biomarker microRNAs in urinary exosomes have relied on databases or profiling to identify CKD markers. Lv et al. investigated the expression levels of miR-29c and miR-21 isolated from urinary exosomes of renal fibrosis (RF) patients to see if urinary exosomes could be used to diagnose RF. They realized that isolating exosomes and observing the upregulation of miR-21 and the downregulation of miR29c is a low-cost and highly sensitive method for diagnosing RF (98). In an investigation on the use of urine exosomes to diagnose Type 2 Diabetic Nephropathy (T2DN), a decrease in urinary exosomal miR-29c-5p and miR-15b-5p and an increase in let-7c-5p were the predictors of type 2 DN (99). Furthermore, bioinformatics analysis has revealed that the upregulation of miR-30a, miR-133b, and miR-342 in urine exosomes is a marker of T2DN (100). The elevation of urine exosomal miR-21, a non-invasive biomarker of CKD, has a deleterious influence on renal function (101). Another example is the reduction of miR-200b in non-proximal renal tubule-derived urinary exosomes as a biomarker for the diagnosis of renal fibrosis in CKD patients (102). Moreover, miR-451 increases as an early response to renal cell injury in CKD patients (103).
2.6. Therapeutic Utilization of MSC-derived Exosomes in Kidney Disease
Anti-apoptotic, antioxidant, anti-fibrotic, anti-inflammatory, and immunomodulatory properties of MSCs highlight their therapeutic potential. As a result, MSC-derived exosomes mimic immunomodulatory and cytoprotective functions of their parent cell and transfer regenerative information to injured cells or tissues (55).
2.6.1. MSC-derived Exosomes in AKI Treatment
Tomasoni et al. used cisplatin to induce AKI in mouse proximal tubular epithelial cells (PTECs). They observed the proliferation of injured PTECs after transferring human bone marrow MSC-derived exosomes containing insulin-like growth factor-1 (IGF-1) (104). In cisplatin-induced AKI mice, Zhou et al. reported that human umbilical cord MSC-derived exosomes increased cell proliferation by activating the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway, reducing blood urea nitrogen (BUN) and creatinine (Cr) levels, tubular protein casts, and proximal epithelium necrosis via anti-apoptotic actions, and acted as an antioxidant (105). Zhu et al. found that exosomes produced by human bone marrow-derived MSC exosomes enriched with miR-199a-3p had an anti-apoptotic effect on renal ischemia/reperfusion injury, one of the most common causes of AKI, in rat models. By activating the ERK and AKT pathways, miR-199a-3p decreased the expression of Semaphorin 3A (Sema3A), cleaved caspase 3, and pro-apoptotic Bcl-2-associated X (Bax) protein, and increased the expression of B-cell lymphoma-2 (Bcl-2) (106). Wang et al. investigated the effects of human umbilical cord MSC-derived exosomes (hucMSC-Ex) on preventing cisplatin-induced nephrotoxicity. They found that pretreatment with hucMSC-Ex increased the light chain 3B (LC3B) autophagosome marker expression in renal proximal tubule epithelial cells by inhibiting the mammalian target of rapamycin (mTOR) pathway. As a result, inflammation and apoptosis reduced, and renal regeneration improved (107). Lindoso et al. used a renal ischemia/reperfusion injury induced by ATP depletion model and incubated them with MSC-extracellular vesicles to investigate the role of MSC-derived extracellular vesicles in changing the miRNAs expression of renal PTECs. They discovered that by modulating the miRNA profile of PTECs, MSC-extracellular vesicles reduced the expression of genes involved in hypoxia, apoptosis, and cytoskeleton reorganization, such as caspase 7 (CASP7), caspase 3 (CASP3), SHC (Src homology 2 domain-containing) transforming protein 1 (SHC1), and SMAD4 (108). Zhang et al. investigated the anti-oxidative role of extracellular vesicles derived from human Wharton's Jelly mesenchymal stromal cells (hWJMSC) in AKI rat models. Cell apoptosis, serum neutrophil gelatinase-associated lipocalin (sNGAL) level, and oxidative stress decreased after hWJMSC-derived extracellular vesicles were treated. Antioxidant activity increased by the augmentation of the Nrf2/antioxidant response element (ARE) pathway and heme oxygenase-1 (HO-1) expression (109). Jia et al. demonstrated that 14-3-3 ζ containing hucMSC-ex enhanced autophagy via binding to autophagy-related protein 16L (ATG16L) and prevented nephrotoxicity in cisplatin-induced AKI rat models (110). Shen et al. investigated the protective impact of MSC-derived exosomes in ischemia/reperfusion-induced kidney damage animal models by administering C-C motif chemokine receptor-2 (CCR2)-enriched BMMSC-exosomes. It has been demonstrated that binding CCR2 to the C-C motif chemokine ligand-2 (CCL2) protein reduces CCL2 concentration and recruitment of macrophages/monocytes for inflammation in renal injury (111). Lin et al. investigated the synergistic effect of adipose-derived mesenchymal stem cell (ADMSC) and ADMSC-derived exosome in acute ischemia/reperfusion injury models. They concluded that ADMSC-derived exosomes protect the kidney by decreasing oxidative stress, inflammation, fibrotic, and apoptotic biomarker levels while increasing anti-apoptotic and angiogenesis biomarker levels (112).
2.6.2. MSC-derived Exosomes in CKD Treatment
The regenerative effect of MSC-derived exosomes was studied in pigs with renal artery stenosis due to CKD. According to the findings, MSC exosomes enriched with interleukin-10 (IL-10) reduced inflammation by lowering the pro-inflammatory cytokines interleukin-1 (IL-1) alpha, IL-1 beta, and tumor necrosis factor-alpha (TNF-alpha), as well as medullary oxygenation and fibrosis (113). Furthermore, researchers used genetically engineered MSCs that overexpressed miRNA-let7c and delivered its exosomes to mice with unilateral ureteral obstruction. Exosome miR-let7c reduced fibrosis and downregulated fibrotic genes such as collagen IV1, α-SMA, transforming growth factor-beta receptor I (TGF-βR1), and (TGF)-β1 (114). Choi et al. investigated the efficacy of kidney mesenchymal stem cell-derived exosomes in unilateral ureteral obstruction (UUO) mouse models with peritubular capillary (PTC) rarefaction. They observed that endothelial-to-mesenchymal transition (EndoMT) and apoptosis reduced while endothelial cell proliferation increased. Furthermore, exosomes inhibited macrophage infiltration (F4/80 positive) and kidney fibrosis (115). Another study found that MSC-derived exosomes, which contain pro-angiogenic genes and proteins, improve renal function and recovery by promoting angiogenesis, reducing microvascular architecture, and endothelial cell apoptosis (116). Furthermore, human adipose-derived MSC (hAD-MSC) exosomes prevented AKI progression to CKD by activating renal tubular Sox9. In C57BL/6 mice, treatment with hAD-MSCs-secreting exosomes increased tubular epithelial cell (TEC) Sox9 and inhibited TEC transformation to a pro-fibrotic phenotype induced by TGF-1. There was also TEC proliferation, reduction of renal ischemia, hypoxia, subsequent fibrosis, and infiltration of inflammatory cells (117). Despite the numerous benefits of MSC-derived exosomes in CKD regeneration, exosomes derived from human embryonic MSCs had no regenerative effects on in vitro rat models of CKD induced by 5/6 nephrectomy (SNX) combined with L-NNA and a 6% NaCl diet (118).
According to a study of the paracrine effects of bone marrow-derived MSCs and MSC conditioned medium on DN regeneration, exosomes improved DN by suppressing abnormal infiltration of bone marrow dendritic cells, renal fibrosis, and epithelial-mesenchymal transition (EMT) and protecting tubular endothelial cells (119). In another study, bone marrow-derived MSC-exosomes improved renal function and morphology in DN rats by upregulating autophagy markers LC3II and Beclin-1 while downregulating mTOR and fibrotic marker expression (120). In a DN rat model, exosomes derived from urine mesenchymal-like stem cells inhibited the apoptosis of podocytes and tubular endothelial cells. They also reduced urine volume and albumin excretion while increasing glomerular endothelial cell proliferation. These exosomes contain factors for promoting vascular regeneration and cell survival, such as transforming growth factor-β1, angiogenin, and bone morphogenetic protein-7 (121).
3. Conclusions
In conclusion, the protective structure of the exosome saves its cargo from degradation; thus, the molecular cargo of exosomes can play essential roles in the early diagnosis and treatment of kidney disease. However, there are not enough studies and clinical trials in this field to ensure the efficacy of MSC-exosomes in kidney treatment. There are also difficulties in selecting faster and more effective methods for isolating and purifying exosomes. Limitation of exosomal biomarkers for kidney diagnosis in the laboratory and insufficient information about the reference value of biomarkers imply that their diagnostic standard is still in its infancy. As a result, accurate detection and precise conformation of biomarker indices are required to improve exosome diagnosis application. Furthermore, our future goals are advancements in the use of MSC-exosomes in cell-free-based kidney therapy to alleviate disease pain and reduce treatment side effects in patients.