1. Background
Opioids such as morphine remain the first choice for treating severe acute and chronic pain in clinics (1). However, the long-term use of morphine has adverse side effects, including tolerance and dependence, limiting pain management using the drug, and increasing the risk of drug addiction (2-4). According to research, the repeated use of morphine induces complex neuroadaptive mechanisms in the central nervous system (CNS), leading to drug tolerance and dependence (5-8). Alterations at the gene and protein expression levels with mu-opioid receptors and the synaptic, cellular, and circuit levels may underlie drug tolerance and dependence (3, 9, 10). Tolerance to morphine also involves alterations in the endogenous opioid peptides (11).
Opioid receptors, including mu, delta, and kappa receptors, are expressed throughout the brain, especially in areas associated with pain and reward processing (12). These receptors are the active sites of endogenous opioid peptides and exogenous painkillers such as morphine (13, 14). Among opioid receptors, the mu-opioid receptors are mainly expressed in mesocorticolimbic areas (15). Morphine via binding to presynaptic opioid receptors in pain pathways suppresses the transmission of nociceptive signals and contributes to opioid-induced antinociception (16, 17). Besides, opioid receptor activation throughout cortical, limbic, and midbrain structures modulates emotional response to pain and is responsible for opioid-induced cognitive disorders (18, 19).
There are three different precursors for endogenous opioid peptides, including proopiomelanocortin (POMC), prodynorphin (PDYN), and proenkephalin (PENK) which produce the main opioid peptides in the body, including β-endorphin, dynorphins, and enkephalins, respectively (20). It has been shown that long-term morphine utility can induce modifications in the homeostasis of the PENK-containing neurons localized predominantly in the substantia nigra, periaqueductal grey, hypothalamus, hippocampus, and striatum, which may affect emotional responses to pain (21, 22). Besides, PDYN-expressing neurons are mainly distributed in the CNS areas associated with pain processing, especially in the spinal cord, brain stem, hypothalamus, and other limbic system areas (23). According to research, the utility of exogenous opioid ligands for inhibiting pain signals affects the number of opioid receptors and influences the endogenous opioid peptides (24). On the other hand, the number of opioid peptides and receptors can influence the effects of exogenous agonists, including morphine, and vice versa (25).
2. Objectives
We aimed to examine possible alterations in the expression of the precursor proteins of endogenous peptides, including Pdyn and Penk, and mu-opioid receptor (Oprm1) at the gene expression level within the mesocorticolimbic pathway, including the midbrain, striatum, hippocampus, prefrontal cortex (PFC), and hypothalamus after an eight-day regimen of frequent morphine treatment.
3. Methods
3.1. Subjects
In the present study, we used male Wistar rats with an average weight of 280 ± 20 g at the beginning of the experiments. The animals were kept in an animal house at a constant temperature (22 ± 2 °C), appropriate humidity (50 - 60 %), and a 12 h light/dark cycle, with illumination beginning at 7:00 (a.m.). The animals had free access to animal feeding pellets and tap water. The international guidelines for using laboratory animals were followed, consistent with the guidelines of the National Academy of Sciences Institute for Laboratory Animal Research (2011). The protocol of the current experiment was also accepted by the Research Ethics Committee (REC) at the University of Kurdistan (IR.UOK.REC.1398.021).
3.2. Repeated Morphine Treatment
Morphine sulfate was a product of Temad as part of Daroopakhsh Co. (Tehran, Iran) and was liquified in saline immediately before use. Morphine tolerance and dependence were induced according to previous reports at our lab (26, 27). Sixteen rats were randomly separated and allocated into two groups (n = 8 per group), including a control saline-treated group and a morphine-treated group using appropriate software (28). Rats in the control group received saline injections (1 mL/kg) subcutaneously, while rats in the morphine-treated group received morphine at a dose of 10 mg/kg. The drug treatments were done twice a day with eight hours intervals for eight consecutive days.
3.3. Brain Dissection
Four rats from each experimental group were randomly subjected to gene expression examination in the midbrain, striatum, PFC, hippocampus, and hypothalamus. On day 8 of the treatment schedule, all animals in both experimental groups received their treatments with 10 min intervals on the morning session, and then each rat was decapitated two hours after the saline or morphine injection. Then, the whole brain was quickly removed from the skull, and the above-mentioned brain areas were bilaterally dissected on an ice-chilled clean surface (29-31). Each tissue sample was moved to a tube, flooded in liquid nitrogen for fast freezing, and then stored in a -80 freezer before total RNA extraction.
3.4. Real-time Polymerase Chain Reaction
Ten milligrams of tissue samples were subjected to total RNA extraction using a High Pure miRNA Isolation Kit based on the manufacturer’s protocol (Roche, Germany). A product of Thermo Fisher Scientific (USA) and its provided manual protocol with the kit was used for complementary DNA (cDNA) synthesis. A LightCycler 96 system was used for performing real-time polymerase chain reaction (RT-PCR) (Roche, Germany). Each biological sample from each rat was examined in triplicate technical repeats. The PCRs were done in 20 µL volumes, consisting of 2 µL mixture of gene-specific primers (5 µM), 8 µL cDNA (4 ng/µL), and 10 µL MasterMix (Yekta Tajhiz Azma Co., Tehran, Iran) (32). The thermal cycling initiated with a pre-incubation step at 95˚C for 30 s, followed by 40 cycles of two-step amplification (denaturation at 95˚C for 5 s and annealing/extension at 60˚C for 30 s), and terminated after melting and cooling phases. The gene expression level was evaluated using the Livak (2-ΔΔCT) method (33). The sequences of the gene-specific primers are summarized in Table 1.
| Gene Symbol | Sequences (5′-3′) | Amplicon Size (bp) |
|---|---|---|
| Gapdh | F: AGTGCCAGCCTCGTCTCATA | 77 |
| R: GGTAACCAGGCGTCCGATAC | ||
| Oprm1 | F: CGATTCCAGAAACCACATTTCA | 66 |
| R: TGTTCGTGTAACCCAAAGCAAT | ||
| Penk | F: GAAGACAGGACTCCCCAAGG | 90 |
| R: GCATTCTGTCTTCCTGGAGGT | ||
| Pdyn | F: AGGATGGGGATCAGGTAGGG | 80 |
| R: CTTAAGCTTGGGGCGAATGC |
Abbreviations: Gapdh, glyceraldehyde-3-phosphate dehydrogenase; Oprm1, Mu-opioid receptor 1; Penk, proenkephalin; Pdyn, prodynorphin.
3.5. Statistical Analysis
The Shapiro-Wilk test approved the normal distribution of the data. The Brown-Forsythe test was applied for assessing variance equality. An independent t-test was used to compare gene expression data between experimental groups. The statistical significance level was set at P < 0.05, and analyses were done using GraphPad Prism version 9.0 software (San Diego, California, USA).
4. Results
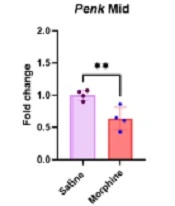
4.1. Penk, Pdyn, and Oprm1 Gene Expression Downregulated in the Midbrain After Frequent Morphine Injections
The qPCR results in the midbrain revealed that morphine treatment to induce analgesic tolerance and dependence significantly downregulated Penk [t (6) = 3.8, P < 0.01], Pdyn [t (6) = 5.94, P < 0.01], and Oprm1 [t (6) = 3.76, P < 0.01] gene expressions compared to the control group (Figure 1).
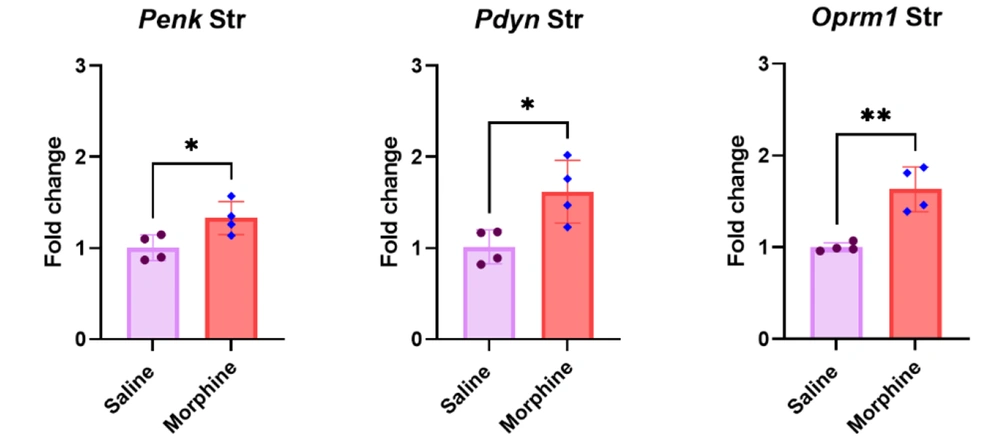
4.2. Repeated Morphine Treatment Significantly Increased Penk, Pdyn, and Oprm1 Gene Expressions in the Striatum
The results of the qPCR in the striatum revealed that the continued administration of morphine for eight days significantly upregulated Penk [t (6) = 2.8, P < 0.05], Pdyn [t (6) = 3.1, P < 0.05], and Oprm1 [t (6) = 5.1, P < 0.01] gene expressions compared to the control group treated with saline (Figure 2).
Effect of long-term morphine injection on Penk, Pdyn, and Oprm1 gene expressions in the striatum. Data are shown as mean ± SD; individual data (n = 4) in each group are also seen on each bar. Penk, proenkephalin; Pdyn, prodynorphin; Oprm1, Mu-opioid receptor 1; and Str, striatum. (* P < 0.05 and ** P < 0.01)
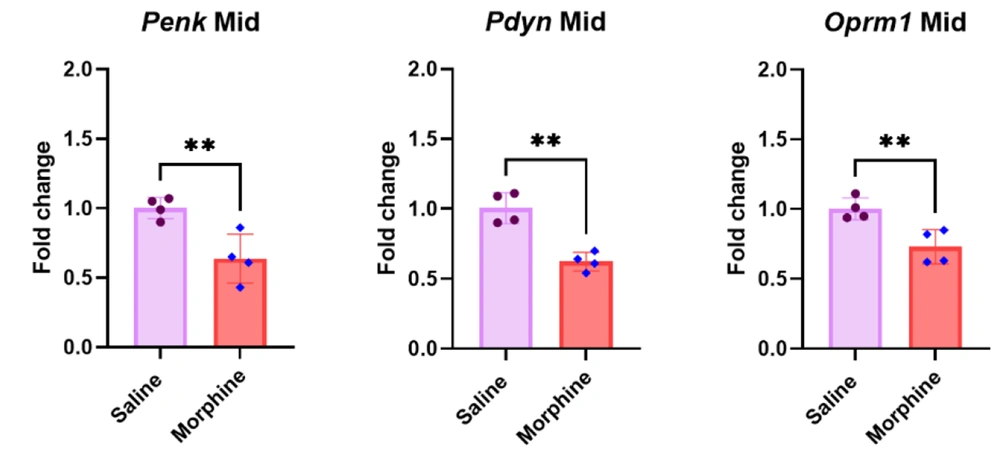
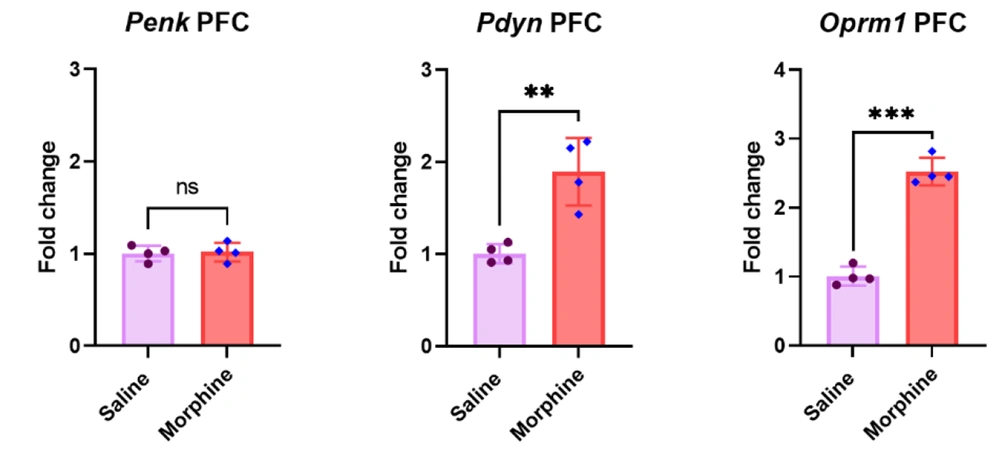
4.3. Pdyn and Oprm1 but not Penk Gene Expressions Upregulated in the PFC After Frequent Morphine Injections
The qPCR results in the PFC revealed that eight days of morphine injection significantly upregulated Pdyn [t (6) = 4.7, P < 0.01] and Oprm1 [t (6) = 12.5, P < 0.001] gene expressions compared to the control group treated with saline. However, no significant difference was observed in the Penk gene expression in the PFC between the saline and morphine-treated groups [t (6) = 0.2, P > 0.05] (Figure 3).
Effect of repeated morphine treatment on Penk, Pdyn, and Oprm1 gene expressions in the PFC. Data are shown as mean ± SD; individual data (n = 4) in each group are also seen on each bar. Penk, proenkephalin; Pdyn, prodynorphin; Oprm1, Mu-opioid receptor 1; and PFC, prefrontal cortex. (ns: non-significant, ** P < 0.01 and *** P < 0.001)
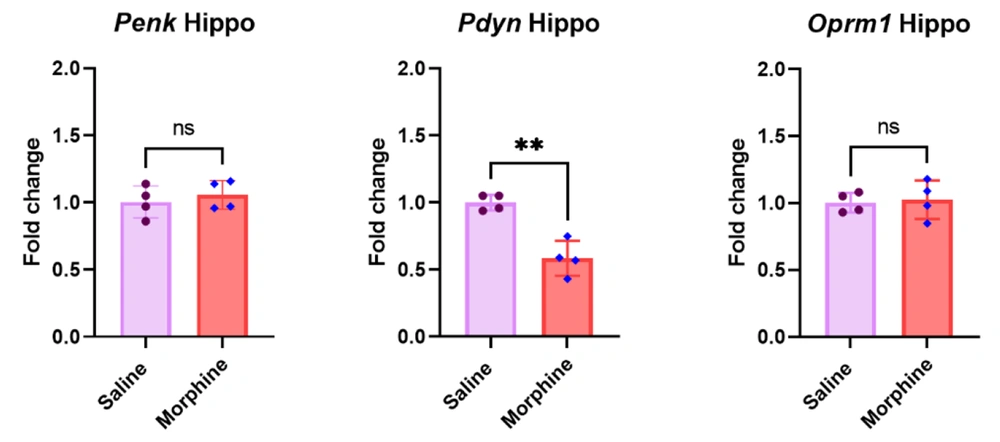
4.4. Repeated Morphine Injections Significantly Decreased Pdyn But Not Penk and Oprm1 Gene Expressions in the Hippocampus
The qPCR results in the hippocampus revealed that frequent morphine treatment significantly downregulated Pdyn gene expression [t (6) = 5.8, P < 0.01]. However, there were no significant differences in the mRNA levels of Penk [t (6) = 0.7, P > 0.05] and Oprm1 [t (6) = 0.3, P > 0.05] in the hippocampus between the experimental groups after morphine treatment (Figure 4).
Effect of frequent morphine injection on Penk, Pdyn, and Oprm1 gene expressions in the hippocampus. Data are shown as mean ± SD; individual data (n = 4) in each group are also seen on each bar. Penk, proenkephalin; Pdyn, prodynorphin; Oprm1, Mu-opioid receptor 1; and Hippo, hippocampus. (ns: non-significant and ** P < 0.01)
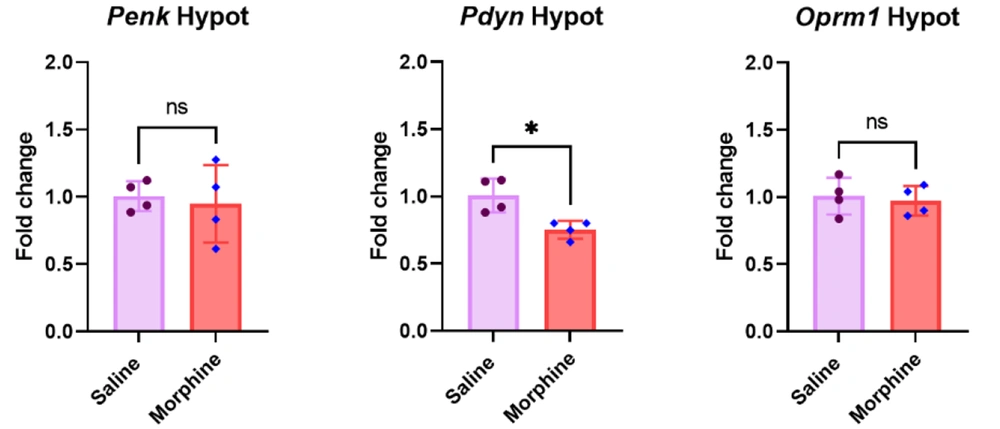
4.5. Repeated Regimen of Morphine Significantly Decreased Pdyn But Not Penk and Oprm1 Gene Expressions in the Hypothalamus
The qPCR results in the hypothalamus revealed that eight days of morphine treatment significantly downregulated Pdyn gene expression [t (6) = 3.6, P < 0.05] compared to the control group. However, the results showed no significant between-group differences in the mRNA levels of Penk [t (6) = 0.36, P > 0.05] and Oprm1 [t (6) = 0.4, P > 0.05] in the hypothalamus (Figure 5).
Effect of frequent morphine treatment on Penk, Pdyn, and Oprm1 gene expressions in the hypothalamus. Data are shown as mean ± SD; individual data (n = 4) in each group are also seen on each bar. Penk, proenkephalin; Pdyn, prodynorphin; Oprm1, Mu-opioid receptor 1; and Hypo, hypothalamus. (ns: non-significant and * P < 0.05)
5. Discussion
We previously showed that frequent morphine injections for eight successive days induced morphine analgesic tolerance and dependence in rats (26, 27). Long-term drug abuse reorganizes cellular and molecular patterns, especially in the brain regions associated with reward and pain processing, which, in turn, alters the responsiveness of the brain to the abused drugs (34, 35). The repeated utilization of opioids such as morphine decreases drug effectiveness known as drug tolerance, limiting its usefulness in medicine and increasing the risk of drug addiction (3). A dominant hypothesis in morphine addiction is that it recruits neuronal circuits and neurotransmitters responding to natural rewards and gradually alters their functions (36, 37). The mesocorticolimbic dopaminergic system has been introduced as a core system in drug addiction. Besides, the internal opioid systems are also involved in the hedonic assessment of natural reinforcements and play a role in the harmful effects of abused drugs (38). The effects of many abused drugs, including morphine, depending on their binding to mu-opioid receptors, support the idea that this type of receptor is a possible molecular entry to drug addiction (39).
The present experiment revealed significant decreases in endogenous peptides, including Penk, Pdyn, and Oprm1, at the mRNA level in the midbrain after a schedule of long-term morphine treatment. On the contrary, the present results revealed significant upregulations in Penk, Pdyn, and Oprm1 mRNA levels in the striatum in rats receiving morphine compared to rats treated with saline as the control group. The current experiment results also revealed that Pdyn and Oprm1 expressions significantly increased in the PFC, but no significant modification was detected in Penk expression after long-term morphine treatment compared to the control group. There were no significant alterations in Penk and Oprm1 expressions at the mRNA level in the hippocampus and hypothalamus. However, Pdyn expression significantly diminished in the hippocampus and hypothalamus in the morphine-treated group compared to the control group.
Interestingly, the present results revealed a region specificity pattern for alteration in the Penk, Pdyn, and Oprm1 expressions at the mRNA level after prolonged use of morphine in some brain areas involved in reward and addiction. It is suggested that the alterations in Penk, Pdyn, and Oprm1 gene expressions precede some other neuroadaptations that may participate in the progress of morphine side effects, including tolerance and dependence. Different reports have indicated that frequent morphine treatment has adverse effects on memory, pain perception, and anxiety-like behaviors in animal models (27, 40). Therefore, one may propose that morphine may alter cellular and molecular processes underlying these behaviors in relevant brain areas such as the midbrain, striatum, hippocampus, PFC, hypothalamus, and some other brain areas.
The midbrain includes the periaqueductal grey matter (PAG) that plays a key role in tolerance to the antinociceptive effects of morphine (9). In addition, the midbrain contains the ventral tegmental area (VTA) and substantia nigra involved in drug reinforcement and addiction via sending dopaminergic projections to forebrain structures (41). The direct action of morphine on mu-opioid receptors located on GABAergic interneurons in the midbrain disinhibits the dopaminergic projecting neurons and increases dopamine levels in the ventral striatum and PFC, which, in turn, mediates positive reinforcement of the drug (42). Therefore, mu-opioid receptors in the midbrain are gateways to morphine tolerance and drug addiction (39, 43). Accumulating data have shown that frequent morphine treatment induces phosphorylation and downregulation of mu-opioid receptors, which, in turn, causes endocytosis of membrane receptors to attenuate the increased input signaling (44-46). Therefore, the decreased Oprm1, Penk, and Pdyn mRNA levels in the midbrain are consistent with the downregulation of opioid receptors and peptides in the reports cited above. The decreased mRNA levels of Penk, Pdyn, and Oprm1 in the midbrain detected in the current study may be due to the negative feedback of the midbrain neurons to the frequent administration of morphine and the increased activation of mu-opioid receptor signaling. There are conflicting reports regarding Penk and Pdyn expressions after morphine treatment in different brain areas. In particular, repeated morphine administration in mice significantly upregulates the mRNA level of Pdyn, but Penk expression remains unchanged in the striatum (47). In another study, chronic injections of morphine for 14 days increased the mRNA level of Pdyn in the rat striatum (48). Some differences in methodology, such as different subjects and duration of repeated treatment between different studies, may explain the differences in the expression of endogenous opioid peptides.
Endogenous enkephalins mediate reinforcing actions but dynorphins, in contrast, produce the aversive states of at least some substances of abuse (49). In particular, the effects of dynorphin-like peptides on kappa-opioid receptors during the injection of a diversity of addictive drugs are aversive and act to prevent the reinforcing properties of those drugs and develop tolerance (50). Kappa-opioid receptors placed on dopamine terminals also disrupt dopamine release (51). It has been shown that the repulsive effects of dynorphins are performed by their actions on presynaptic kappa-opioid receptors in the ventral striatum via inhibiting the dopamine release (52). Besides, some reports indicate that dopamine D2 receptor blockade increases, whereas D2 receptor stimulation decreases the striatal proenkephalin mRNA level (53). It has been recently reported that dopamine released from dopaminergic terminals in the striatum is rapidly controlled by local regulatory mechanisms such as the dynorphin system (54). Based on the data mentioned above, the present results may demonstrate that the frequent injection of morphine and the subsequent overflow of dopamine in the target areas may gradually increase Pdyn expression, which, in turn, increases Penk expression via dopamine release reduction and dopamine D2 receptor mechanism. The release of endogenous dynorphins in the prefrontal cortex disrupts cognition (55). Therefore, the increased expression of Pdyn in the PFC may be a possible explanation for cognitive impairments in morphine-dependent animals reported elsewhere (18, 56).
Chronic morphine treatment in rats decreased the Pdyn mRNA level in the hypothalamus (57). It has also been shown that Penk and Pdyn expressions decrease in the rat hippocampus after the administration of dextromethorphan, which is an opioid-like drug (58). Therefore, Pdyn downregulation in the hippocampus and hypothalamus in the current experiment is consistent with previous reports. However, we did not detect significant changes in Oprm1 and Penk gene expressions in the hippocampus and hypothalamus. A possible explanation may be that alterations in these molecules may occur at the post-transcriptional level. One limitation of this study is that we only examined the alterations in Pdyn, Penk, and Oprm1 at the mRNA levels without evaluating their protein levels. However, the alterations at the mRNA level may be a precedence for subsequent modifications in protein levels of the examined molecules, but further experiments are needed to confirm their exact roles in the brain adverse effects of morphine.
5.1. Conclusions
The present experiment showed that Pdyn, Penk, and Oprm1 gene expressions follow a region-specific pattern in the mesocorticolimbic areas after prolonged administration of morphine. These results confirm that chronic morphine treatment affects not only Oprm1 expression but also endogenous opioid peptides Pdyn and Penk in the brain reward circuits. These alterations may result in new physiological setpoints outside the normal range in the function of opioidergic systems, which, in turn, may affect animal behaviors. Therefore, the opioidergic systems may be considered a potential complementary drug target in pain management and addiction prevention.