1. Background
Glioblastoma (GBM, known as glioblastoma multiforme) is one of the most common and also most aggressive solid tumors in adults. It has a highly invasive behavior leading to difficulties in complete therapy and poor prognosis, with only a survival period of 12 - 15 months after diagnosis and more than 5% survival in less than 5% of patients (1-7). In spite of the gradual evolution over time in differential diagnosis and treatment approaches, there has not been a significant increase in patient survival (6-10). The unique biology, anatomical challenges, and heterogeneous nature of the brain microenvironment, such as the blood-brain barrier (BBB), different immune cell types, and secreted cytokines district this organ from others, provide more difficulty in the way of treatment of all CNS tumors and cause the development of therapeutic resistance (8-12).
As a non-negligible factor, the accumulating evidence has supported the tumor microenvironment (TME) for drug resistance and the focus of research because combating metastasis instead of being on cancer-center is on TME (13). The TME factors can trigger inter- and intra-cellular signaling, mediating tumor initiation, progression, metastasis, and response to therapies (14). Therefore, understanding the fundamental glioblastoma biology and the molecular landscape beyond GBM metastasis, which are influenced and regulated by the TME factors, is highly crucial for better diagnosis and therapy.
The experimental data have shown that the glycosylation pattern of cells is disrupted in tumor cells (15). Glycosylation is a posttranslational modification resulting in protein sialylation, and its pattern disruption causes tumor progression and metastasis. This glycosylation influences the glycoproteome profile of the cells, which can be considered as microenvironmental cues acting in triggering special signaling promotion in cells toward disease evolution. Recent data have shown that alterations in the pattern of glycosylation play the main role in aggressive cell behavior determination (16). It is reported that most considerable therapeutic insults of edge cells originate from tumor core cells (17); thus, finding inducer factor-mediated and regulated extra- and intracellular mechanism interactions involved in drug resistance is a crucial step in combating tumor metastasis. Sialylation pattern disruption can lead to intervening in interactions of receptor-ligand and also might cause evading from immune surveillance due to masking tumor cell surface and cover antigens (16, 18-24). Furthermore, high sialylation and disruption of the sialylation pattern of the glycan on the cell membrane are one of the main causes to enable cancer cells to evade therapeutic approaches (25, 26). Though high expression of glycoproteins in body fluids is a useful indicator enabling the assessment of cancer, and change in glycosylation of them is a hotspot property for cancerous cells (27).
It was acclaimed that in immunotherapy, the best approach for increasing the potentiality of antibody-drug entrance into the brain is a modification of glycan- sialylation pattern, which is performed through inhibition of efflux (28, 29). Scientists have reported the association of extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation reduction with a reduction in the sialic acid content of cell surface (18). It is interesting to note that environmental factors can influence ERK1/2 phosphorylation status with a cross-talk with other signaling mechanisms related to the growth factors, cytokines, or mitogens triggering cell differentiation, proliferation, migration, or death (18, 30). Hypersialylation pattern of cells is verified in different cancers, such as glioma, neuroblastoma, and lung cancer (31, 32). As a major effect of platelet-derived growth factor (PDGF)-D, phosphorylation of ERK1/2 has been introduced in recently published data (33).
1.1. PDGF-D
Platelet-derived growth factor (PDGF) structure is composed of the disulfide-bonded dimeric isoforms that are involved in growth and survival procedures; its function includes embryonic development, proliferation, migration, survival, and chemo-taxis of cells (34). Besides, it has a key role in healing wounds and repairing damage to the blood vessel walls. The PDGF family consists of four classes, including PDGF-A, -B, -C, and –D, also known as Iris-expressed growth factor (34, 35).
In 2017, it was reported that PDGF family genes were up-regulated in glioblastoma, and owing to their pivotal roles in glial cell dedifferentiation into stem cells, intratumoral stimulation of angiogenesis, lymphangiogenesis, and immunosuppression have been considered as drivers of glioblastoma tumor progression and metastasis, as well as potential therapeutic targets; but different expression patterns have been diagnosed based on the location of the brain tumor (36).
1.2. NRP1
Neuropilin is a type I transmembrane glycoprotein receptor for semaphorins and different ligands related to angiogenesis. It is involved in signal transduction and plays a crucial role in organ development. It affects the growth and guidance of axons through binding to class III semaphorins and vascular endothelial growth factor (VEGF) (37, 38).
1.3. ERK1/2
The ERK1/2 expressed and activated not only in glioblastoma cells but also in the areas around the tumor, transduces growth factor signal to the nucleus, which can induce proliferation, differentiation, and motility (39). It has been demonstrated that the decrease in the proliferation and migration of the glioma cells was due to the inhibition of MEK/ERK1/2 signaling (40). Associated with molecules involved in cell-cell adhesion, ERK signaling could be involved in all stages of tumorigenesis, such as initiation, growth, and progression (40).
2. Methods
2.1. Cell Line and Cell Culture
The astrocytoma cell line 1321N1 was purchased from the Cell Bank of the Pasture Institute (Tehran, Iran) and cultured in DMEM low glucose medium (Gibco, USA), supplemented with 10% fetal bovine serum (Gibco, FBS, USA), and 1% penicillin (5000 U/mL)-streptomycin (5000 mg/mL). It was incubated at 37°C with 5% CO2 and a humidity of 95%.
2.2. Cell Viability
In previous experiments, our research group tested the influence of sialic acid on cellular toxicity. We incubated 1321N1 cells with different concentrations of Sialic acid for 24 h and determined its' cytotoxic ability by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), a tetrazole assay. No significant change in cell morphology was disclosed after Sialic acid treatment of up to 300 uM. EC50 and IC50 in the presence of sialic acid were determined for 1321N1 cells; therefore, we decided to use concentrations of 300 - 1000 uM sialic acid for all further experiments (concentration of sialic acid was selected based on previous studies) (41).
2.3. Morphometric Analysis by VEGF and VEGFR1 Staining
The 1321N1 glioblastoma cells were seeded in a 12-well plate (0.5 × 105 cells per well) and cultured for 24 hours under a standard condition. The cells were treated with sialic acid (300 uM, 500 uM, and 1000 uM) for 24 hours. The media was removed, and the cells were washed using PBS, followed by fixation with ethanol. The fixed cells were then washed with PBS. VEGF (sc-7269) and VEGF-R1 (sc-271789) antibodies were used in 1:50 dilutions for cell staining based on the manufacturer’s protocol (SANTA CRUZ BIOTECHNOLOGY, INC). The staining intensity was evaluated by fluorescence microscopy. For this purpose, cell staining was photographed at 20X magnification after immunohistochemistry using a fluorescence microscope (BM-600 LED Epi-fluorescent; Germany).
2.4. Western Blot
Forty micrograms of the extracted protein were separated by 10% SDS-PAGE gel and then transferred to PVDF membranes. Then, 5% dried milk was used to block the membrane for two hours, and they were then incubated overnight with primary antibodies at the temperature of 4°C. The primary antibodies used in our study were anti-NRP1 (1:200, sc-5307), anti-PDGFD (1:50, sc-137029), anti-ERK1,2 (1:200, sc-514302), anti-p-ERK1,2 (1:200, sc-16981-R), and anti-β-actin (1:300, sc-47778). The secondary antibody is the appropriate horseradish peroxidase-conjugated antibody (Anti-Rabbit, 1:1000), and then enhanced chemiluminescence detection reagents (ECL advanced reagents; Santa Cruz Biotechnology, INC) were used for visualization of the protein bands, prior to visualization on a Kodak-o-mat film according to the manufacturer’s protocol.
2.5. Statistical Analysis
All experiments were run in duplicate. The data obtained from two independent experiments were expressed as mean ± SD (standard deviation). The statistical analyses were performed using a two-tailed student t-test. A P < 0.05 was considered significant.
3. Results
3.1. Cell Viability
In our previous study, the metastatic effect of sialic acid was confirmed depending on the time and concentration manner. MTT assessment revealed that sialic acid treatment could significantly increase cell viability and hyper-cellularity. The enhanced/stimulated and the inhibited concentration of sialic acid used to treat 1321N1 cell lines after 24 h was found to be 490 and 1298 μM, respectively (The results of the MTT assay for 1321N1 were reported in our previous work) (41).
3.2. Glioblastoma 1321N1 Cell Staining for VEGF/VEGFR-1
3.2.1. VEGF-Expression
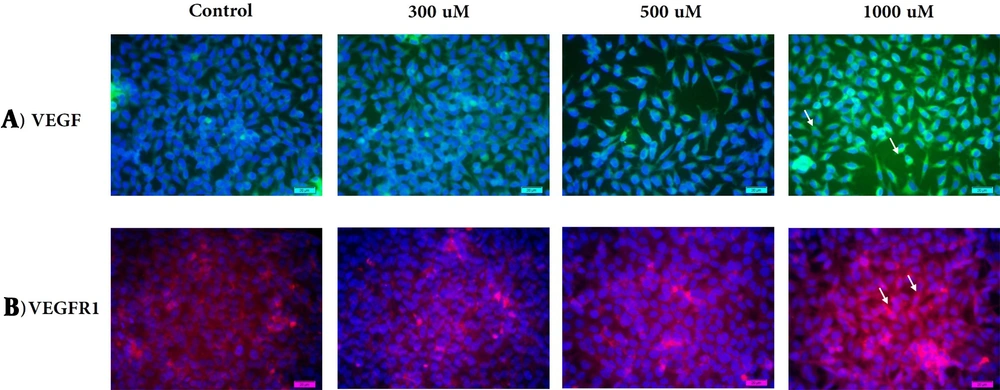
VEGF-positive cells were found in all concentrations, but they were weakly positive at a low concentration and highly positive at a high concentration of sialic acid. There was a significant difference in staining intensity between the three groups compared to the control group. Intracellular staining demonstrated VEGF localization around the nucleus and did not change localization status during induction of metastasis (Figure 1A).
Immunofluorescence staining. VEGF-positive cells (green; Alexa 488) and VEGF-R1- positive cells (red; Alexa 564) treated with sialic acid at 300, 500, and 1000 uM, respectively. Cells are DAPI-counterstained (blue nuclei). The VEGF/VEGF-R1 staining showed intracytoplasmatic and cell surface loci for VEGF around the nuclei (→) (A) and VEGF-R1 on the cell surface (→) (B).
3.2.2. VEGF Receptor1-Expression
VEGF-R1 is a 180 kDa glycoprotein detected in cells treated with a high concentration of sialic acid. VEGF-R1 expression increased significantly during increasing in sialic acid concentration. We observed an almost significantly lower expression of VEGF-R1 in glioblastoma 1321N1 exposed to 300 uM of sialic acid, which may reflect resistance to induction. Considering that metastasis, at least in the initial steps, can be independent of VEGFR1 expression, preclinical and clinical studies verified VEGFR-1 involvement in metastatic behavior. Its activation can trigger the release of immune cytokines, which favors cancer immune escape called concomitant. Expression of VEGFR may affect cell chemotaxis and extracellular invasion. In this study, we observed a high expression of VEGFR-1 in glioblastoma cells treated with 1000 uM of sialic acid, the so-called angiogenic switch, and metastatic spreading. We have detected cells with high expression level of VEGFR1 on the cell surface (Figure 1B).
3.3. PDGFD is Up-regulated in the Presence of Sialic Acid
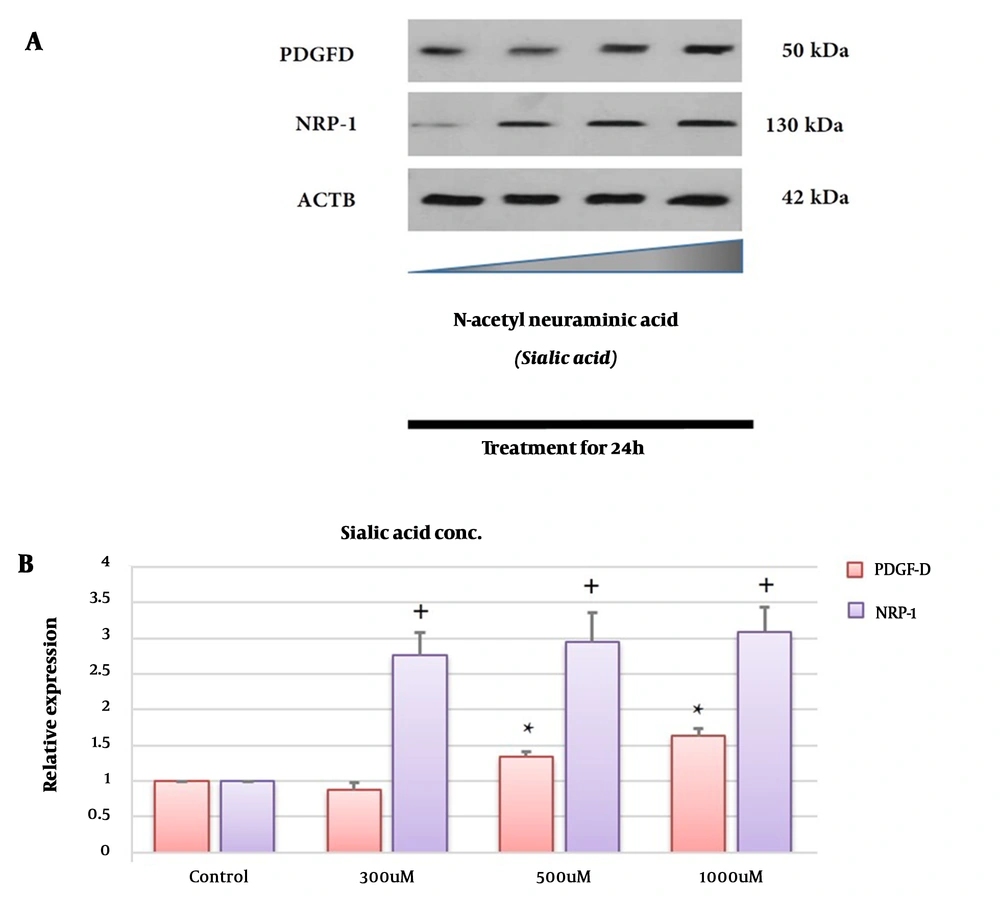
PDGFD expression was tested by Western blotting at the protein level. As shown in Figure 2A and B, the PDGFD level, after treatment with sialic acid, was significantly up-regulated in 1321N1 compared to untreated cells (P < 0.05). These observations suggest that PDGFD was up-regulated approximately 1.6 times in the 1321N1 cell line after treatment with high concentrations of sialic acid.
Effects of sialic acid on the protein expression of glioblastoma (GBM). GBM cells were treated with or without sialic acid for 24 h. The cells were harvested, centrifuged, and analyzed by Western blotting using antibodies specific to PDGF-D and NRP-1. The density ratio of proteins to β-actin from two independent experiments is shown as relative expression (* or +: Significance difference compared to PDGF-D and NRP-1, respectively, P < 0.05). The bands of the western blot analysis were quantified using a densitometer (A and B).
3.4. NRP1 is Up-regulated in the Presence of Sialic Acid
NRP1 expression was tested by Western blotting at the protein level. As illustrated in Figure 2A and B, the NRP1 expression was significantly up-regulated in 1321N1 after treatment with sialic acid compared to untreated cells (P < 0.05).
These observations suggest that NRP-1 up-regulated approximately 3.1 times in the 1321N1 cell line after treatment with high concentrations of sialic acid. Interestingly, NRP1 expression up-regulated approximately two times in the presence of sialic acid related to PDGF-D (Figure 2B).
3.5. ERK1/2 and p-ERK1/2 Are Up-regulated in the Presence of Sialic Acid
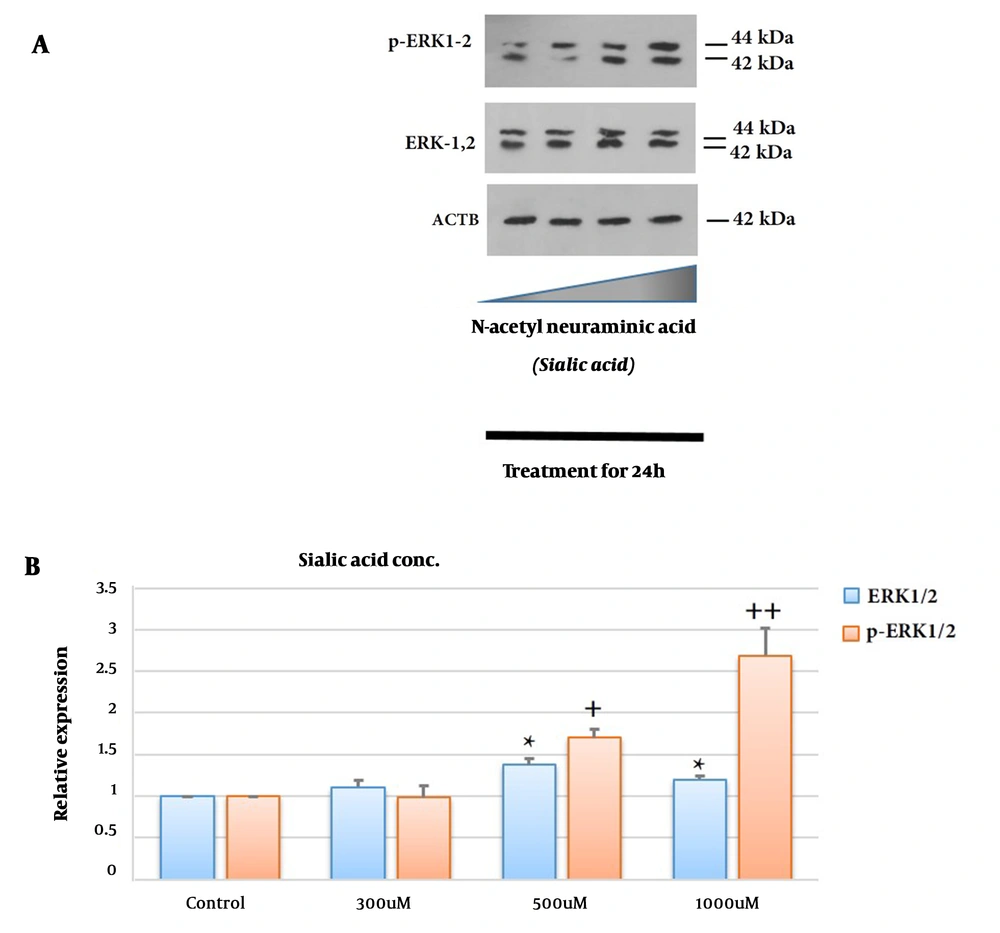
ERK1/2 and p-ERK1/2 expression levels at the protein levels were tested by Western blotting (Figure 3). ERK1/2 and p-ERK1/2 expression at the protein levels significantly up-regulated in 1321N1 after treatment with sialic acid compared to untreated cells (P < 0.05 and P < 0.01, respectively, at a high concentration of sialic acid). These observations suggest ERK1/2 and p-ERK1/2 up-regulated in the 1321N1 cell line after treatment with increasing sialic acid concentrations. Interestingly, their expression up-regulated by approximately 1.5 and 2.7 times, respectively, in the presence of a high concentration of sialic acid. Up-regulation of p-ERK was more than PDGF-D but not as high as NRP-1 (Figure 3B).
Effects of sialic acid on the ERK1/2 and p-ERK1/2. Glioblastoma (GBM) cells were treated with or without sialic acid for 24 h. The cells were harvested, centrifuged, and analyzed by Western blotting using antibodies specific for ERK1/2 and p-ERK1/2. The density ratio of proteins to β-actin from two independent experiments is shown as relative expression (* or ++: Significance difference compared to ERK1/2 and p-ERK1/2, respectively; P < 0.05 and P < 0.01). The bands of the western blot analysis were quantified using a densitometer (A and B).
4. Discussion
Based on the experimental data, the cell microenvironment can stimulate cell signaling, which can cause the regulation of tumor angiogenesis, resistance to therapy, and cancer progression (42). Glycocalyx network, as the main factor of the TME, surrounded cells, mediated cell response to the extracellular micro-environment, enabled cells to be masked from immune system detection, and promoted cell metastasis. Therefore, it is critical to identify key factors involved in tumor genesis and progression to determine novel targets for diagnostic and treatment strategies (13-15). Recent data have shown that sialic acid concentration is up-regulated in cancers, such as breast, ovarian, colon, colorectal, etc. (18, 24, 25). As a nexus factor of the architecture of cellular and molecular signaling crosstalk-mediated drug resistance, we hypothesized that sialic acid might contribute to the therapeutic insult (manuscript is prepared; data not shown).
In the previous study, our team determined the EC50 and IC50 of sialic acid for GBM cells; however, it should be noted that our observations confirmed that sialic acid increased cell viability and survival and had no significant cytotoxicity effect on cell lines (43). Sialic acid might trigger molecular mechanisms to induce the inflammatory response by the immune system (41, 44) and increased proliferation and invasion, including MMPs/TIMPs ratio imbalance associated with down-regulation of miR-218 and up-regulation of NF-kB (41). Also, it can up-regulate pro-inflammatory cytokine expression, including IL-1β, IL-6, IL-23, IFN-γ, and TNF-α, and induce EGF, EGFR, MAPK, and RAS signaling, which may cause drug resistance (manuscript under publication).
Different molecular, cellular, and microenvironmental mechanisms are involved in drug resistance (45). Also, the pro-angiogenic inducer, due to its crucial roles in tumor growth and metastasis, including invasion and extravasation, is an excellent therapeutic target in several types of cancers (46). Considering VEGF-dependent alterations as the main mechanism involved in therapeutic resistance (46), we used an immuno-fluorescent study to show that sialic acid could induce VEGF/VEGF-R1 expression (Figure 1A and B).
The VEGF or PDGF, so-called PAF, is the main inducer and mediator factors, respectively, which are involved in angiogenesis (47). The goal of this study was to survey the sialic acid effect on PDGF-D expression, which its combined expression with VEGF-E has a pivotal role in the induction of the angiogenic processes (48).
Confirming the importance of the PDGF-D signaling in human malignancies, because of its involvement in the regulation of different stages of cancers, introduced PDGF-D signaling as a therapeutic target (33). Considering the VEGF-PDGFR binding challenges paradigm of the uni-family ligand-receptor binding, understanding the molecular scenario beyond these novel cross-family interactions can provide a novel approach for overcoming resistance to anti-angiogenic drugs and increasing the significant role of growth factor signaling in glioblastoma. We confirmed that sialic acid treatment induced PDGF-D expression (Figures 2A and B). These data are in concordance with other data suggesting that if we want to improve the therapeutic efficacy, we must reduce the sialic acid content in malignant cells.
Also, recent data have revealed that binding PDGF-D to the neuropilin 1 (NRP-1) induced the PDGFRβ-NRP1 complex formation and colocalization, which has been confirmed in fibroblasts and translocates NRP1 to cell-cell junctions in endothelial cells. NRP-1 can induce PDGF-D-PDGFRβ signaling and can be an intercellular communicator in the vascular wall (49). Following treatment with sialic acid, NRP-1 expression significantly increased concentration-dependently confirming the previous data about sialic acid effects on proliferation, migration, and metastasis and showed a strong correlation with VEGF expression in this study and empowered our theory about the fact that sialic acid as microenvironmental agent can play an important role in triggering metastasis and drug resistance.
On the Other hand, the ERK1/2 pathway (classical MAPK) is induced in glioma cells upon treatment with sialic acid, which is consistent with other data on the PDGF-D and NPR-1 expression. Besides, in concordance with other data about cell proliferation assays by MTT, scratch-assay, cell cycle assessment (manuscript is prepared; data not shown), and VEGF/VEGFR expression (Figure 1A and B), the proliferative and mitogen effect of sialic acid is more confirmed.
4.1. Conclusions
We conclude that sialic acid can induce growth factors, particularly PDGF-D, which is associated with an increase in NPR-1 expression and ERK1/2 activation. Thus, we should target and regulate sialic acid content or its transporter to achieve better results from therapy. For this purpose, scientists have suggested vaccine production for sialic acid reduction. Consequently, our results theoretically and experimentally provide a basis for understanding the mechanism of the sialic acid effect on GBM cells and provide an opportunity for therapeutic targets in GB therapy.