1. Introduction
Various cells secrete exosomes, such as B lymphocyte cell lines, mast cells, dendritic cells, endothelial cells, stem cells, neuronal cells, endothelial cells, and cancer cells (1). Exosomes are released by diseased or viable cell types in a continuous way or by activation into interstitial spaces or body fluid. It is notable that these exosomes are released by cells in vitro (2). As shown by studies, exosomes can be used to develop cell-free vaccines for a variety of diseases (3). Studies have also mentioned their role in normal physiological conditions (such as immune response, lactation, and neuronal function) and pathophysiological conditions (such as the progression and development of liver diseases, cancer, and neurodegenerative diseases) (4). Given the cell origin, exosomes control morphogen transporters in creating polarity in differentiation and development (5). A significant necessity in tissue engineering is inducing lineage-specific differentiation of stem cells reliably and predictably. Generally, biomaterial selection and properties depend on this factor. Growth factor delivery systems are usually used to induce lineage-specific differentiation of stem cells. Using growth factors such as bone morphogenetic factor 2 (BMP2) for clinical applications has been used by the US Food and Drug Administration (FDA). Still, available clinical uses of growth factors have notable ectopic interactions and side effects. Several problems have been reported caused by BMP2, which are major concerns for clinicians (6). To develop biomimetic approaches and solve the issue of using growth factors and complicated controlled release mechanisms, the chance of using exosomes specific to cell type has been studied (7). In general, it is believed that exosomes act as a mediator of cellular homeostasis through secreting cellular waste (8).
Research projects on exosomes have been chiefly on cancer biology and immunology (9). Still, following the findings regarding their role in messenger RNA (mRNA) and microRNA (miRNA) transference (10), many researchers have worked on using them in regenerative medicine. Studies have shown that exosomes enhance the proliferative capacity of epithelial and mesenchymal cells through the mitogen-activated protein kinase (MAPK) pathway (11). Exosomes extracted from endothelial progenitor cells, endothelial cells, and mesenchymal stem cells demonstrate proangiogenic characteristics explained by the presence of miRNAs localized in them (12). Eventually, exosomes are recognized as the driving force of immunomodulatory effects of mesenchymal stem cells (MSCs) by triggering the secretion of anti-inflammatory cytokines and accelerating the formation of M2 macrophages (13). Despite the findings supporting exosomes’ capabilities to be used in regenerative medicine, their capacity to cause lineage-specific differentiation of stem cells is not supported by reliable evidence. Moreover, the role of using exosomes secreted from one partition in the general biology of the recipient cell requires further studies. Our hypothesis states that exosomes from differential cells can cause lineage-specific differentiation of native MSCs.
2. Exosomes Biogenesis and Release
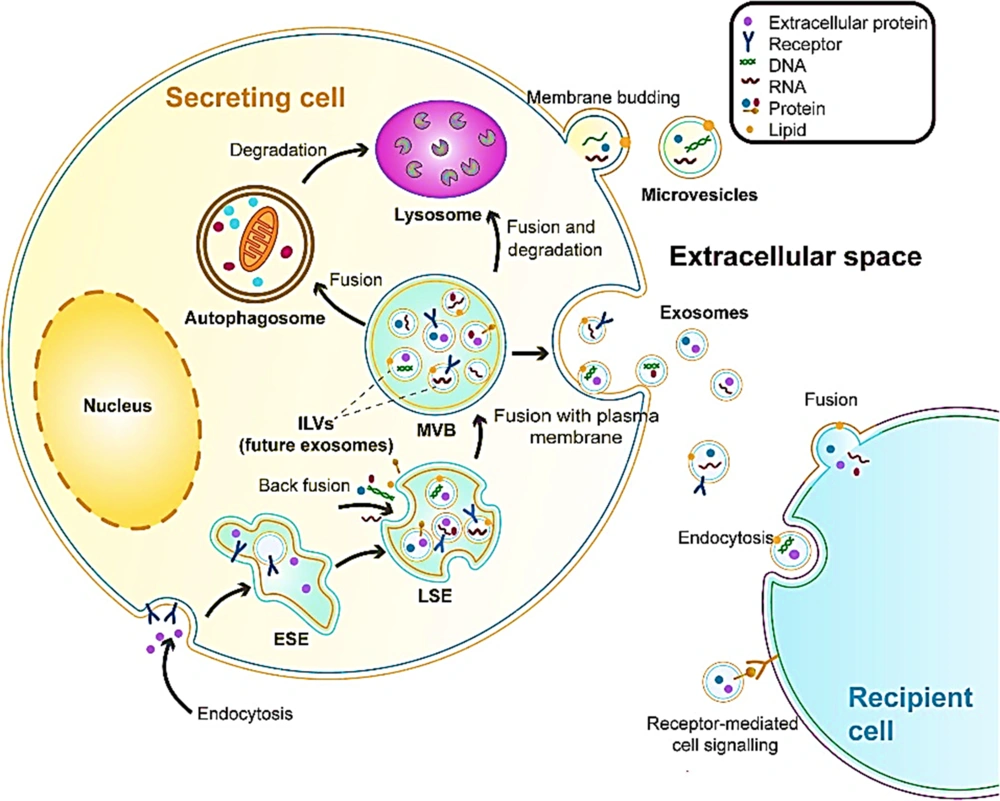
The source of exosomes is the internal budding of the plasma membrane that happens along with endocytic internalization. This is a mature process starting from an early endosome via interaction with the Golgi complex to the late endosome, where the bilayer membrane secretes intraluminal vesicles (exosomes). Multivesicular bodies contain future exosomes. These membranous exosomes contain proteins, mRNA, DNA, miRNA, lipids, and other molecules from the cytoplasm of their parent cell (Figure 1). In addition, multivesicular bodies can be sent to lysosomes from degradation or fuse with the plasma membrane and release exosomes via exocytosis (14). A variety of cell types secrete exosomes. They can be extracted from cultured cells and body fluids such as salvia, urine, blood plasma, breast milk, ascites fluid, bronchoalveolar lavage fluid, synovial fluid, amniotic fluid, nasal mucous, cerebrospinal fluid, bile, and semen. A variety of materials are found in extracellular vesicles (EVs), such as sugars, proteins, lipids, and nucleic acids (including miRNA, mRNA, and DNA), which are found inside the vesicles protected from nucleases and proteases (15). Therefore, exosomes have a role to play in the intercellular exchange of RNA and proteins. They also play a role in regulating angiogenesis or immune responses. It is possible to translate mRNA in exosomes into recipient cells and also silence the expression of the recipient cell’s gene and alter their phenotype. Exosomes also stimulate specific signaling pathways. The biogenesis of EVs and the content can be determined using cellular sources that are sensitive to the status of cells and changes in the environment. A higher release of exosomes has been seen in acidic pH, hypoxia, oxidative stress, and heat shock (16).
3. Exosomes in Inducing Odontogenic Differentiation
Exosomes obtained from different types of stem cells can be used for therapeutic purposes for differentiation and angiogenesis (17-19). Notably, exosomes of normal dental pulp stem cells (DPSCs) can cause regeneration of dental pulp-like tissue (20) and demonstrate the capacity to trigger angiogenesis in vivo and in vitro (21). DPSCs extracted from damaged periodontal teeth (P-DPSCs) demonstrate the capability to retain pluripotency and regeneration capabilities; however, they are not extensively used for therapeutic purposes. There is evidence that they can keep the potency and regenerative potential; however, their use as a treatment is minimal. Moreover, MSCs secrete more exosomes with inflammation than under normal conditions (22). Consistently, P-DPSCs secrete a higher level of exosomes compared to DPSCs obtained from periodontally healthy teeth (H-DPSCs). In addition, another study showed that exosomes of lipopolysaccharide (LPS)-preconditioned DPSCs demonstrated a higher ability to promote Schwann cell proliferation, odontogenic differentiation, and migration compared to exosomes of normal DPSCs (23).
A new solution to treat dental pulp or periapical complications of permanent teeth with open apices is regenerative endodontic procedures (REPs) (24). Stem cells of the apical papilla (SCAP) are extracted from the dental papilla and then differentiated into dental pulp cells and primary odontoblasts that generate root dental pulp and dentine (25, 26). Exosomes are the principal regulator of the paracrine activity of stem cells; thus, they affect the function of recipient cells (27). The authors in (28) introduced SCAP exosomes (SCAP-Exo) into the root fragment with bone marrow MSCs (BMMSCs) and then transplanted them into mice with immune deficiency. Their findings supported the presence of dental pulp-like tissues, and the freshly formed dentine was added to the dentine in the root canal. Next, the effects of SCAP-Exo on dentinogenesis of BMMSCs were examined. Their findings indicated a significant increase in the expression of the gene and protein in dentine sialophosphoprotein and mineralized nodule formation in BMMSCs exposed to SCAP-Exo. In short, BMCSCs endocytosed SCAP-Exo and enhanced the specific dentinogenesis. Using exosomes of dental stem cells can create the potential treatment for dentine-pulp complex regeneration in REPs (29).
4. Exosomes in Promoting Differentiation of Exocrine Cells into the Pancreatic Lineage
Diabetes is one of the most serious health issues worldwide (30), and the only available treatment is pancreas transplantation or islet. Still, transplantable tissues are highly scarce (31, 32). This has led researchers to find new sources of cell differentiation. Along with small molecule-based and genetic approaches, exosomes can induce cellular differentiation using their cargo, such as miRNA (33-35). Mandal et al. (36) introduced a protocol based on chemicals to differentiate mouse embryonic fibroblasts (MEFs) into β-like cells. They also used mouse insulinoma (MIN6)-derived exosomes with and without specific molecules to facilitate differentiation in β-like cells. The differentiation outcomes worked and expressed pancreatic genes (such as Nkx6.1 and Pdx1) and insulin 1 and 2. The authors showed that the combination of exosomes with small molecule was the most efficient in differentiating MEFs. The results also showed that exocrine cells could easily be differentiated into β-like cells using exosomes and exosome-identified miRNAs. It is possible for diabetic patients to use a new way of differentiation using exosome-identified miRNAs. It appears that exosomes can be differentiated from induced pluripotent stem cells (iPSCs) into insulin-producing cells (IPCs). By selectively packaging specific miRNA into exosomes and transferring them to recipient cells, it is possible to regulate post-transcriptional gene expression that influences the differentiation of stem cells (37). Islet cell-specific genes are targeted by miRNAs indirectly or directly, leading to degradation or translation repression of specific mRNAs. Therefore, it can regulate islet cell differentiation and maturation (38-41). A key element in the function of miRNA is argonaute-2 (Ago2), which also plays a role in the packaging of miRNAs into exosomes (42-44). There are reports that the role of exosomal miRNA depends on Ago2 obtained from the donor cells rather than the recipient cells (45, 46).
Guo et al. examined the role of exosomes obtained from a murine pancreatic β-cell line and tried to determine signature exosomal miRNAs on iPSCs differentiation. They reported that iPCSs cultured using an exosome-containing medium had a higher expression of MAFA, insulin 2, Neuroud21, insulin 1, NGN3, and Nkx6.1 compared to the control iPSCs. About 52.7% of the differentiated cells demonstrated the expression of insulin at the middle stage. Through transplanting 21-day-induced IPCs into type I diabetes (T1D) mice, glucose tolerance was improved efficiently, and hyperglycemia was controlled partially (47). The potential for treatment decreased significantly in T1D mice injected with iPCSs cultured with siAgo2 exosomes. The findings showed that exosome differentiation induced by iPSCs in function cells essentially depended on the specific miRNAs encased in exosomes. By finding candidate miRNAs, we can have a deeper understanding of the molecular interactions in the differentiation of stem cells and achieve a deeper insight into new regulator mechanisms that control the differentiation of iPCSs into IPCs (47).
5. Exosomes and the Differentiation of Stem Cells into Neurons
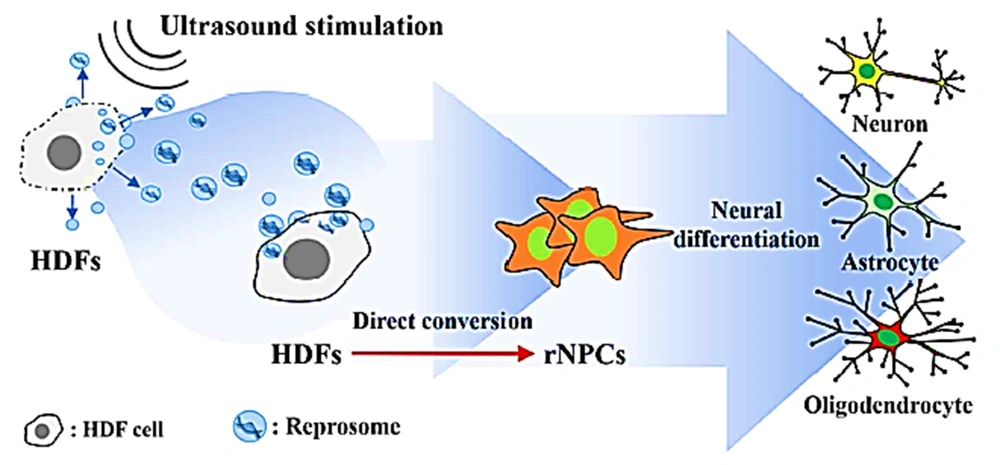
A reliable source to restore damage to neural tissues is to convert somatic cells directly into neural progenitor cells (NPCs). NPCs induction from human dermal fibroblasts (HDFs) is used along with integrating exogenous proneuronal transcription factors without or with chemical elements or with a mixture of chemical elements (48-51). To activate exosome release, stress conditions are used, including hypoxia, heat shock, mechanical stress, and oxidative stress (52-55). While converting directly appears to be favorable for cellular repair, using xenobiotics still creates challenges for clinical uses (such as lack of efficiency) mainly because of the selective permeability of cell membranes in the process of transmembrane transportation of exogenous material. In addition, the risk of viral vectors of chemicals is notable (56). To solve such challenges, exosomes, as a natural safely transfer cocktails of endogenous material, can be a solution for reprogramming cells efficiently into NPCs. A study reported developing a method to induce NPCs from HDFs using the reprosome-mediated direct conversion of HDFs into NPCs (rNPCs) through treating HDFs using pro-neural reprosomes discharged by HDFs and stimulated using ultrasound and cultured in a neural media. The authors used autologous exosomes with a cocktail of reprogramming factors (reprosomes) to extract NPCs from fibroblasts (57). The obtained fibroblasts underwent ultrasound and were then cultured in neural stem cell medium for 24 hours to trigger the release of reprosomes made of reprogramming agents associated with neural lineage-specific factors and chromatin remodeling.
Following reprosome treatment, fibroblasts were efficiently changed into NPCs (rNPCs) by triggering chromatin remodeling. The process only took 5 days to form 1500 spheroids demonstrating an NPC-like phenotype. NPCs showed proliferation and self-renewal features for weeks and were efficiently differentiated into astrocytes, neurons, and oligodendrocytes in vivo and in vitro (Figure 2). Cellular reprogramming using reprosome as a mediator is an efficient, safe, and simple way to generate autologous stem cells for medical uses (58).
Neural differentiation (57)
6. Exosomes Secreted by Cells and Promote Osteogenesis
An efficient method to regenerate significant bone defects is distraction osteogenesis (DO). Still, the period of treatment is too long, mostly for aged patients (59).
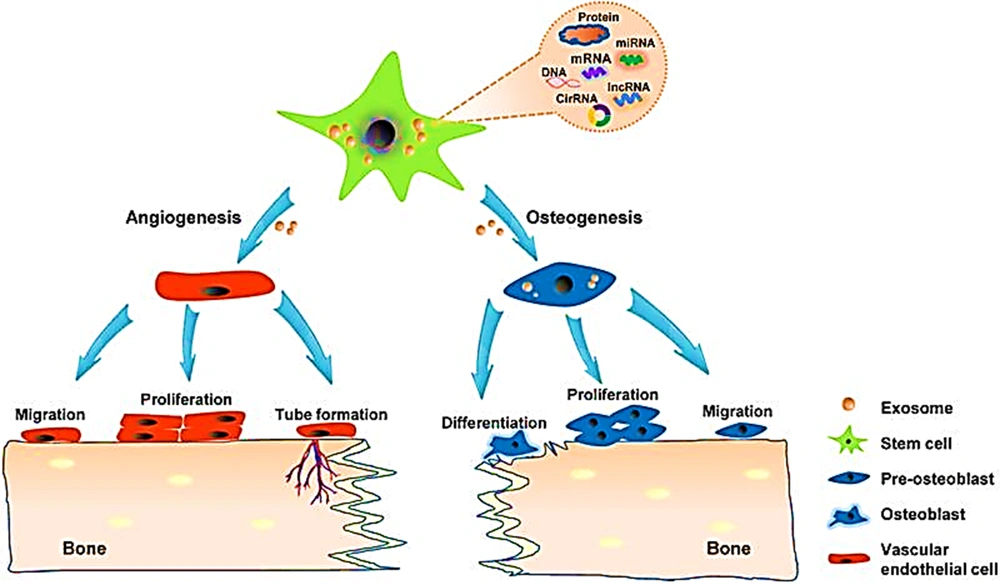
In this study, in vitro findings showed that MSC-Exos could improve osteogenic differentiation (Figure 3) and proliferation of older BMSCs. Rats showed a notable acceleration of bone regeneration after being treated with MSC-Exos. The rats were examined using micro-computed tomography (CT), X-ray, and histological analysis. These findings indicate that MSC-Exos improved DO-mediated bone regeneration in older rats using a higher proliferation of osteogenic capacity in BMCSs. Exosomes demonstrate a remarkable therapeutic ability in bone tissue engineering. Recent research has shown that human adipose-derived stem cells (hADSCs), in the bone regeneration phase, not only take part in osteogenesis through direct differentiation but also through discharging a variety of cytokines and EVs that affect the process. As standard EVs, exosomes seem like a good strategy for bone regeneration therapy (60, 61). Compared to direct autologous transplantation of cells, treatment based on exosomes has benefits such as accurate targeting, stability, low immunogenicity, quickly crossing biological barriers, and low immune reaction (62, 63). The use of hADSCs-Exos for regenerative medicine and tissue engineering is a rich field of research, which is stated by a large number of studies supporting the efficiency of hADSCs-Exos on tissue repair and wound healing in many physiological systems (64). A study indicated that exosomes from controlled media of BMSCs enhanced regeneration of bone in the early stage and improved angiogenesis. This facilitates osteogenesis progression (65).
Exosome and osteogenesis (61)
According to Zhu et al. (66), hADSCs-Exos are prone to be internalized by hADSCs at the parent cells-but not cells of other resources. They argued that this could be due to the homology of the recipient cells. Some lines of evidence suggest that exosomes binding to recipient cells include diverse endocytic pathways such as micropinocytosis, phagocytosis, and endosomal or plasma membrane fusion (67). According to Takeuchi et al., MSC-Exos contain miRNAs that can improve the secretion of VEGF from the recipient cells. This enhances the regeneration of bone (65). In addition, Chen et al. showed that exosomes obtained from miR-375-overexpressing hADSCs improved the regeneration of bone (68).
In short, hADSCs-Exo can be an excellent choice to compensate for the drawbacks of traditional hADSCs therapy in bone repair. It is possible to use strategies to load new bone tissue engineering scaffolds with exosomes shortly.
7. Conclusions
The authors believe that the data reported here can highlight a starting point for others to explore the uses of cell-type exosomes from cultural cells under specific conditions and describe the responses in the target stem cells. As reported by other studies, purified exosomes can be generated in large quantities, lyophilized, and made available commercially to be used with autologous stem cells and other clinical materials such as collagen sponges for regeneration purposes. There is a need for more studies to highlight the differences between primary and subsequent signaling mechanisms triggered by exosomes. This can help us realize the tools in operation. There are several studies on cell-derived extracellular matrix containing biomaterials. It is possible to use exosomes for reconstructing a perfect extracellular environment for reliable and safe differentiation of stem cells.