1. Background
Acetaminophen (APAP) is a commonly used analgesic and antipyretic drug. If APAP is used in high doses, liver necrosis and nephrotoxicity can occur in laboratory animals and humans (1). The APAP induces an animal model of nephrotoxicity to evaluate the efficacy of new therapeutic agents.
Silymarin (SM) is extracted from the Silybum marianum L. Gaertn (milk thistle) seeds. It is a flavonoid with various biological impacts, such as cardioprotective, hepatoprotective, and anticancer (2-5). The SM protects the kidney against toxicants, such as cisplatin, vancomycin, and methotrexate (6-8). However, low aqueous solubility, poor permeability, and extensive metabolism of SM limit its therapeutic usage.
Several protocols, such as incorporating microspheres, liposomes, nanocarriers, and poly(lactic-co-glycolic acid) (PLGA) nanoparticles, improved the bioavailability and solubility of SM. The PLGA is a nontoxic and biodegradable polymer appropriate for tissue engineering, gene therapy, and drug delivery (9). The PLGA encapsulation effectively enhances the hepatoprotective effects of SM (10).
The APAP causes damage to the mitochondrial membrane and releases cytochrome c in response to oxidative stress, causing apoptosis in target cells. The BCL-2 family genes located in the inner mitochondrial membrane regulate the release of cytochrome c (11). Two groups of pro-apoptotic and anti-apoptotic genes exist in the BCL-2 family. The release of cytochrome c from the mitochondria into the cytosol is brought about by the pro-apoptotic proteins, such as Bax. The anti-apoptotic genes, such as BCL-2 and BCL-XL, cause the prevention of the release of cytochrome c into the cytosol and inhibition of apoptosis. Therefore, the ratio of BAX to BCL-2 was used to identify the inducing apoptosis in various cells (12).
2. Objectives
The present study evaluated the effect of nanostructured SM (NSM) against APAP-induced nephrotoxicity and apoptosis.
3. Methods
3.1. Preparation of Nanostructured Silymarin
Loading SM in PLGA was performed by solvent evaporation protocol. In brief, 4% PLGA (w/v) was provided in 1: 4 acetone. Ethyl acetate solution had 0.58% (w/v) SM added to 4 mL of polyvinyl alcohol (PVA) (1% w/v) diluted in water. The prepared nanoemulsion was mixed with 6 mL of 0.5% PVA solution, and the dissolved organic solvents were deleted by a rotavapor. The supernatants were centrifuged at 12,000 rpm for 20 minutes, and the produced pellet was dispersed in deionized water and lyophilized.
3.2. Characterization of Nanostructured Silymarin
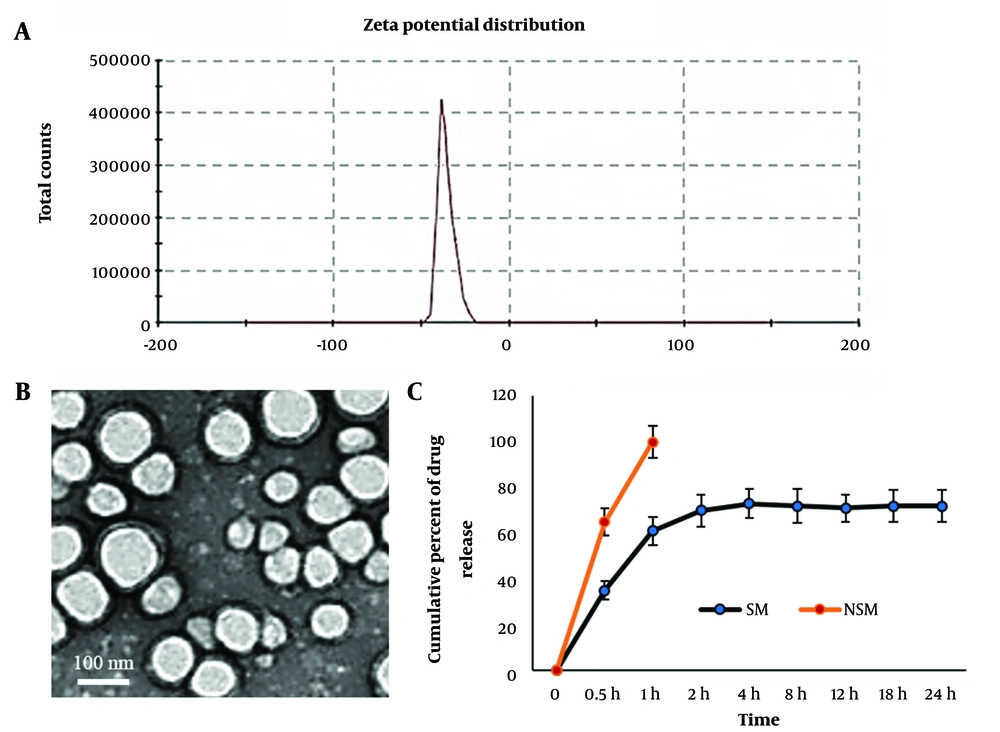
The mean particle size and size distribution of NSM were assessed using a dynamic light scattering device. The charge of the SM-loaded PLGA (Zeta potential) was determined by a Zeta-sizer system (Malvern, UK). The morphology of NSM was examined by transmission electron microscopy.
The entrapment efficiency (EE) was calculated using the following formula:
For the determination of SM release from PLGA, NSM was enclosed in a dialysis bag, placed in 100 mL phosphate-buffered saline, and analyzed spectrophotometrically at 287 nm. The percentage of SM release was calculated by the following formula:
3.2.1. Animals
A total of 48 male NMRI mice (weight range: 20 - 25 g; age range: 6 - 8 weeks) were used in this experimental study. The mice were provided by the Experimental Research Center of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The mice were maintained on a 12-hour-dark and 12-hour-light cycle, with a relative humidity of 50 ± 5% and 22 ± 3°C. The mice had free access to commercial food (pellet) and water. The current study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1393.151).
3.2.2. Experimental Design
The mice were randomly divided into six following groups:
(1) Control group: Received only normal saline.
(2) SM group: Received 5 mg/kg SM for 7 days by intragastric gavage.
(3) NSM group: Received 5 mg/kg NSM for 7 days by intragastric gavage.
(4) APAP-intoxicated group: Normal saline was given for 7 days, and 300 mg/kg of APAP was injected on the 6th day.
(5) SM + APAP group: Received 5 mg/kg of SM for 7 days, and APAP was injected on the 6th day.
(6) NSM + APAP group: Received 5 mg/kg of NSM for 7 days, and APAP was injected on the 6th day.
One day after APAP administration, the animals were anesthetized with ketamine/xylazine, and their blood samples were collected. The mice were then euthanized, and their kidneys were removed in formalin for histological assessments or maintained at -80°C to determine gene expression.
3.3. Biochemical Tests
The blood samples were collected in a heparinized tube and centrifuged. The serums were isolated and stored at -70°C for biochemical tests. The serum concentrations of uric acid, creatinine (Cr), and blood urea nitrogen (BUN) were determined spectrophotometrically using available kits (Sigma-Aldrich, USA).
3.4. Histology Changes
In this study, 6 hematoxylin-eosin-stained sections per mouse were evaluated for histological criteria, including nuclear pyknosis, infiltration of inflammatory cells, brush border loss, and congestion of red blood cells (RBCs). The histological criteria were graded into four categories, namely normal (-), weak (+), moderate (++), or intense (+++). The glomerular diameter was determined by Motic Images Plus 2.0, an image analysis software.
3.5. Real-time Polymerase Chain Reaction
An RNeasy kit was used to isolate the ribonucleic acid of the kidneys, and a complementary deoxyribonucleic acid (cDNA) kit was utilized to convert it to cDNA. The cDNA amplified in polymerase chain reaction (PCR) reaction contained primers (Appendix 1) and SYBR Green. For PCR amplification, a 45-cycle program, including 95°C for 10 seconds, 95°C for 15 seconds, 60°C for 20 seconds, and 60°C for 20 seconds, was used. In this study, the GAPDH gene was used to normalize target genes. The relative expression of genes was investigated using the 2-ΔΔCT method.
3.6. Data Analysis
The data are presented as mean and standard deviation. A one-way analysis of variance followed by a Tukey’s test was used to calculate differences between group means. When the P-value was less than 0.05, the results were deemed statistically significant.
4. Results
4.1. Characterization of Nanostructured Silymarin
The average particle size of SM-loaded PLGA was about 126 nm. In scanning electron microscopy evaluation, NSM had a uniform, discrete, and spherical shape. The EE of NSM was 89.18%. The in vitro drug release profile exhibited an initial burst release until 2 hours and then showed a slow release of SM. The slow release of NSM was continued for 24 hours (Figure 1). Zeta potential results indicated that NSM had good stability for encapsulating SM (Table 1).
| Nanoparticles | Particle Size (nm) | PDI | ZP | EE (%) |
|---|---|---|---|---|
| SM-PLGA | 82.3 ± 5.8 | 0.14 ± 0.05 | -28.4 | 89.18 ± 6.4 |
| Blank-PLGA | 80.13 ± 6.7 | 0.12 ± 0.06 | -27.3 | - |
Abbreviations: ZP, Zeta potential; PDI, polydispersity index; EE, encapsulation efficacy; SM, silymarin; PLGA, poly(lactic-co-glycolic acid).
a Values are expressed as mean ± standard deviation.
4.2. Organ Weight
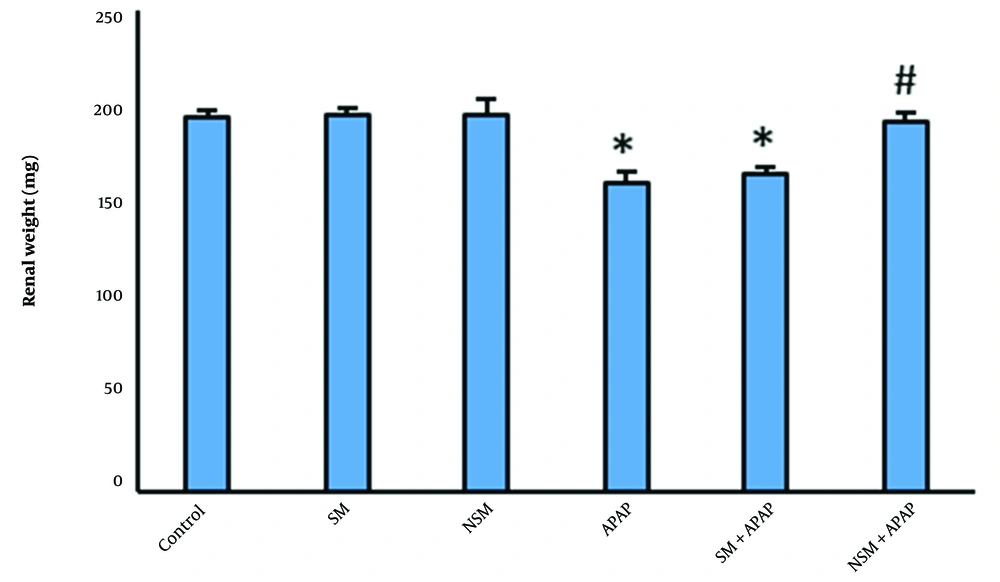
No significant difference was observed in body weight between the control and experimental groups. Organ weight in SM or NSM-treated mice was similar to the controls. Renal weights in APAP-intoxicated mice had significantly reduced, compared to the controls (P < 0.01). The pretreatment of SM slightly increased the renal weights, compared to the APAP-intoxicated animals (P < 0.05). In NSM + APAP-treated mice, the renal weights were significantly higher than in the APAP and SM + APAP groups (Figure 2).
4.3. Biochemical Tests
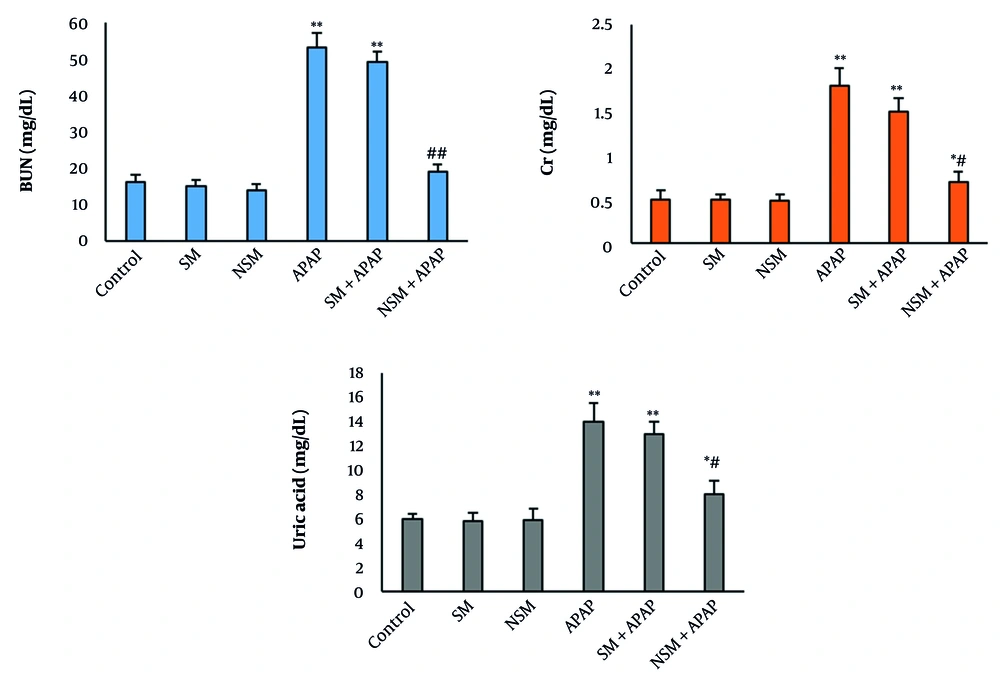
In SM and NSM groups, the serum levels of uric acid, Cr, and BUN did not significantly change compared to the control animals. The serum concentration of the biomarkers significantly elevated in the APAP group (P < 0.001). The pretreatment of SM could not significantly change the biomarker levels compared to the APAP-intoxicated animals. In the NSM + APAP group, the serum level of biomarkers was significantly reduced, compared to the APAP + SM- and APAP-treated animals (Figure 3).
Biochemical tests in various groups (mean ± standard deviation; n = 8) control, silymarin (SM), nanostructured silymarin (NSM), acetaminophen (APAP), SM + APAP, NSM + APAP; * P < 0.05, ** P < 0.01, # P < 0.05, and ## P < 0.01; * and # indicating comparison to the control and acetaminophen groups
4.4. Histology
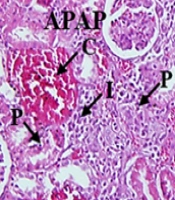
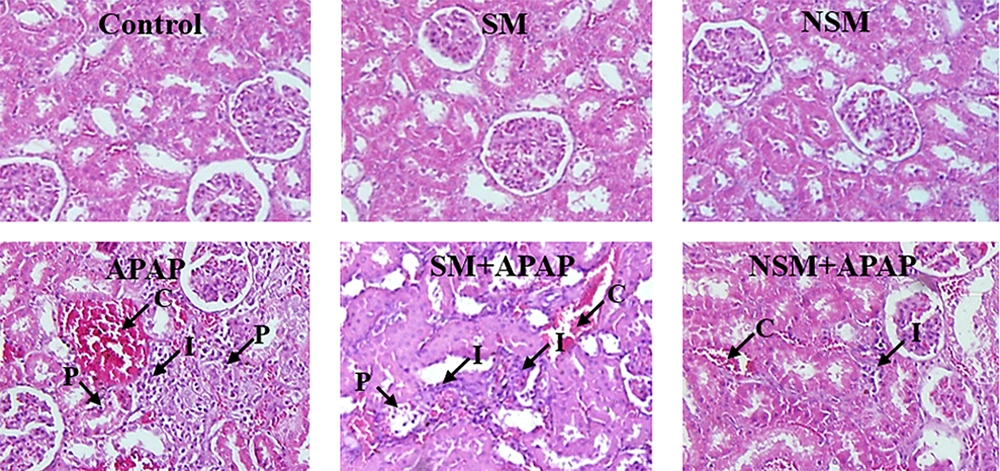
All kidney sections had a normal appearance in the control, SM, and NSM groups. The administration of APAP considerably increased the infiltration of inflammatory cells, congestion of RBCs, and proximal cell damage. Nevertheless, the glomerular diameter was significantly reduced (P < 0.01). The administration of SM + APAP slightly changed the proximal tubule damages (P < 0.05), infiltration of inflammatory cells, congestion of RBCs, and glomerular diameter (P < 0.01), compared to the APAP-intoxicated animals. In the NSM + APAP group, histological criteria significantly reduced, compared to the APAP and SM + APAP groups. The NSM could also increase the glomerular diameter in the APAP-intoxicated mice (Figure 4).
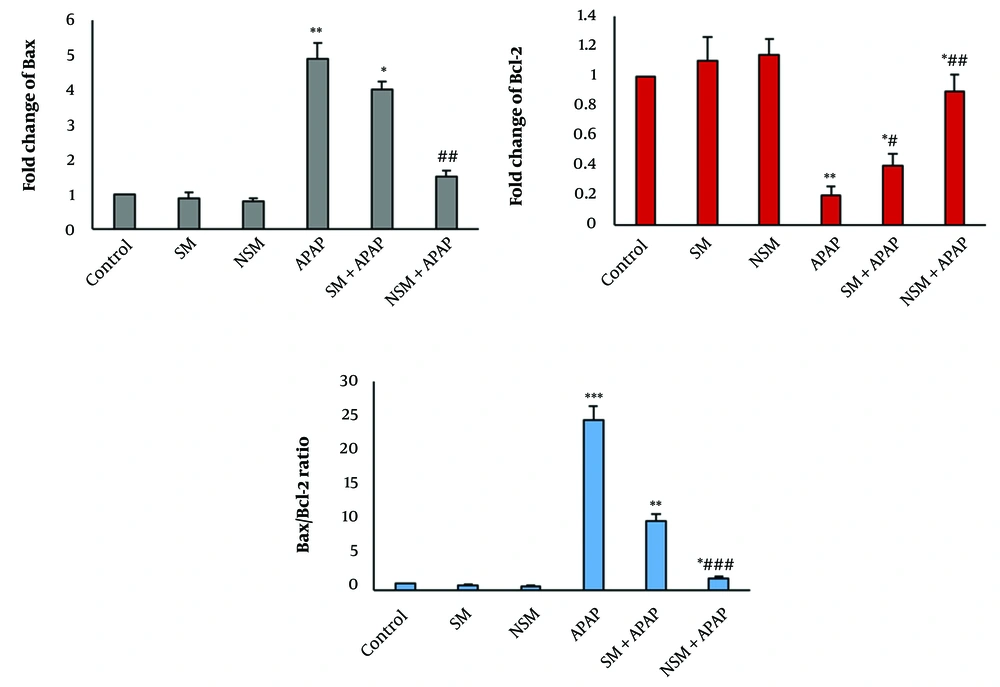
Expression of BAX and BCL-2 in various groups; control, silymarin (SM), nanostructured silymarin (NSM), acetaminophen (APAP), SM + APAP, NSM + APAP (mean ± standard deviation; n = 5); * P < 0.05, ** P < 0.01, *** P < 0.001, # P < 0.05, ## P < 0.01, and ### P < 0.001; * and # indicating comparison to the control and acetaminophen groups
4.5. Real-time Polymerase Chain Reaction
In the APAP group, the expression of the BAX gene in the renal tissue was significantly increased, compared to the control group (P < 0.01). Nonetheless, APAP significantly reduced the expression of the BCL-2 gene. In the SM + APAP group, the expression of the BAX gene in the renal tissue was significantly decreased, compared to the APAP group (P < 0.05). However, the expression of BCL-2 was significantly increased (Figure 5).
Micrographs of hematoxylin-eosin-stained slides of various groups; control, silymarin (SM), nanostructured silymarin (NSM), acetaminophen (APAP), SM + APAP, NSM + APAP; I, infiltration of inflammatory cells; C, congestion of red blood cells; P, damaged proximal tubules; magnifications: X100
In the NSM + APAP group, the expression of the BAX gene in the renal tissue was significantly decreased, compared to the APAP and SM + APAP groups (P < 0.01). Nevertheless, the expression of BCL-2 was significantly increased, compared to the APAP and SM + APAP groups (Figure 5).
The ratio of BAX/BCL-2 in the APAP-intoxicated rats was considerably increased, compared to the controls (P < 0.001). In the SM + APAP group, the ratio of BAX/BCL-2 in the renal tissue was significantly increased, compared to the APAP group (P < 0.01). In the NSM + APAP group, the BAX/BCL-2 ratio in the renal tissue was significantly reduced, compared to the APAP and SM + APAP groups (Figure 5).
5. Discussion
In the present study, SM was successfully encapsulated in PLGA, and this encapsulation could effectively enhance SM’s nephroprotective impacts against APAP-induced renal toxicity in mice. The increased nephroprotective impacts of NSM might be due to the increased bioavailability of SM by PLGA. In a previous study, a liposomal formulation increased the oral bioavailability of SM (13). The SM nanoemulsion attenuates tetrachloride-caused liver damage (14). The NSM, due to its small size, might better penetrate the renal tissues compared to the free SM. Moreover, NSM might escape from macrophages, and its clearance might be prolonged and therefore improve the therapeutic index of SM.
In the APAP-intoxicated mice, an increase in the serum levels of BUN, Cr, and uric acid occurred. The serum levels of these markers are sensitive to any kidney disorder. When the kidney is damaged, these biomarkers, which are inside the proximal cells of nephrons, release into the bloodstream. Therefore, the elevated concentration of these biomarkers indicates proximal cell destruction (15). In this study, NSM effectively ameliorated the serum levels of BUN, Cr, and uric acid. Pretreatment with methanolic extract of SM declined Cr clearance and proteinuria and attenuated proximal tubule injury of rats (16).
In the APAP-intoxicated mice, the increased levels of biomarkers were accompanied by increasing histological criteria. As shown in the results, SM significantly attenuated histological changes induced by APAP. Kumar et al. showed that NSM had more anti-inflammatory action in comparison to the free SM in liver tissue (13). The anti-inflammatory effects of SM have also been reported by Gupta et al. (17). Bektur et al. showed that SM had protective effects against APAP-induced nephrotoxicity in mice (18). Dabak and Kocaman showed that SM ameliorated nephrotoxicity induced by methotrexate in rats. The SM also attenuated kidney cell injury induced by APAP in vitro (8).
As mentioned in the results, kidney weights decreased in the APAP group. It might have been the result of the proximal tubule and glomerular damage. In the APAP-intoxicated mice, the decline in renal weight was accompanied by an increase in the BAX/BCL-2 ratio. This finding indicates that apoptosis is involved in APAP-induced nephrotoxicity, which is in line with the results of previous studies (1, 8). As mentioned in the results, NSM could significantly reduce the BAX/BCL-2 ratio indicating the anti-apoptotic action of NSM. In line with the results of the current study, the anti-apoptotic impacts of SM against cisplatin and aminoglycoside are reported (19). The reduced BAX/BCL-2 ratio in the NSM + APAP group indicated the anti-apoptotic impact of NSM on the renal tissue.
5.1. Conclusions
In this study, SM-PLGA nanoparticles (NSM) were developed to improve the renoprotective effect of SM. The NSM could effectively increase renal weight and reduce apoptosis by decreasing the BAX/BCL-2 ratio in the renal tissue of mice. Further studies are required to prove the anti-apoptotic impacts of NSM on nephrotoxic renal tissues.