1. Background
Opioids have desirable effects in controlling severe acute and chronic pain; however, their prolonged treatment is accompanied by undesirable side effects such as tolerance, dependence, and addiction (1). The ventral striatum is the main part of the mesolimbic reward pathway in which neuroadaptation underlies adverse outcomes of the abused drugs. An increase in dopamine levels in the ventral striatum is mainly implicated in the rewarding and addictive properties of the long-term use of morphine (2). Local neurons in the ventral striatum receive different afferents and express different receptors. Therefore, not only the direct effect of morphine on mu-opioid receptors and its indirect effect via affecting the release of dopamine but also different local regulatory mechanisms in the ventral striatum are involved in subsequent neuroadaptations that are influenced by frequent use of morphine (3).
Further, different studies have reported activating innate immune cells by opioids via binding to toll-like receptor type 4 (TLR4) in the central nervous system, promoting opioid tolerance, dependence, and addiction (4, 5). It has been revealed that morphine, via binding to TLR4, activates the expression of nuclear factor-kappa B (NF-κB), resulting in neuroinflammation through increases in pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin-1β (IL-1β), and IL-6 (5, 6). Based on these findings, some investigators have proposed that antagonists of TLR4 could be used as a new opioid adjuvant strategy to improve opioid analgesic effectiveness and also to oppose opioid-induced tolerance (7, 8).
Functional interactions between opioid and cannabinoid systems in rewarding and analgesic effects have also been demonstrated in several biochemical, molecular, and pharmacological studies (9, 10). Cannabinoid receptor type 1 (CB1R) and MORs have been found in the same presynaptic nerve terminals with shared signaling pathways through a common receptor-mediated G-protein pathway (11, 12). Some evidence also shows that opioids increase the release of endocannabinoids (13). Accumulating evidence shows that not only opioids but also cannabinoids affect immune signaling by influencing TLR-mediated signaling pathways (14, 15). Accumulating data also reveals the involvement of specific microRNAs (miRNAs) in regulating gene expression mediated by the chronic use of morphine (16, 17).
2. Objectives
We aimed to examine the mRNA levels of some of the cell membrane receptors, including MOR, dopamine D1 receptor (DRD1), DRD2, CB1R, CB2R, and TLR4, pro-inflammatory cytokines and their respective receptors, as well as some downstream signaling molecules in the ventral striatum following frequent use of morphine. We also examined the expression of some biologically-relevant miRNAs to morphine tolerance and addiction in the ventral striatum to explore their association with possible alterations in the expression of the examined genes following chronic morphine treatment.
3. Methods
3.1. Subjects
Sixteen four-month-old male Wistar rats weighing 280 ± 20 g were used. Sample size estimation was based on a power analysis method conducted using G*Power-free software (18). Rats were kept under the standard conditions, including constant temperature (22 ± 2°C), 12 h light/dark cycle (light on at 7:00 and off at 19:00), and 50 - 60% humidity. The animals were kept in four per cage and could access enough standard pellet food and water. The study protocol followed the international guidelines for the care and use of laboratory animals (19), which the local ethics committee also approved at the University of Kurdistan (IR.UOK.REC.1398.021).
3.2. Drugs and Experimental Design
Morphine sulfate was a crystal powder produced by Temad (Daroopakhsh Co., Tehran, Iran), dissolved in physiologic saline. Animals were randomly allocated into two experimental groups (n = 8 each) using appropriate software (20). One group of the animals as a control group received subcutaneous (s.c.) injections of physiologic saline (1 mL/kg), and the other group received chronic injections of morphine (10 mg/kg, s.c.) twice daily for eight successive days. The injections were performed by an experimenter who was blind to the experimental design.
3.3. Dissection of the Ventral Striatum
On day 8, two hours following the daily injection of either saline or morphine, each rat was deeply anesthetized with a mixture of ketamine and xylazine (100 and 10 mg/kg, respectively), decapitated using a decapitator (Tajhiz Gostar Omid Iranian, Tehran, Iran), the whole brain was quickly removed from the skull, and the ventral part of the striatum in both hemispheres was finally dissected on an ice-chilled sterile surface (21, 22). The dissected tissues were immediately submerged in liquid nitrogen for fast freezing to save their RNA contents from degradation by cell RNases.
3.4. Gene Expression Study
Total RNAs were extracted from the tissue samples using a commercial kit (High Pure miRNA isolation kit, Roche, Germany). The quality and concentration of the extracted RNAs were examined with agarose gel electrophoresis and a spectrophotometry method (Eppendorf BioPhotometer 6131, Germany), respectively. The presence of the intact bands corresponding to the 28 s and 18s ribosomal RNA on 1% agarose gel electrophoresis and the optimal purity ratios (A260/280 and A260/230) between 1.9 - 2.2 were criteria for including samples in further evaluations. Equal amounts of the total extracted RNA from the biological repeats were reverse-transcribed to complementary DNA (cDNA) using a two-step cDNA synthesis kit (Thermo Fisher Scientific, USA). Real-time PCR was performed using a LightCycler 96 system (Roche, Germany). Each biological repeat in each experimental group (n = 8) was examined in triplicate technical repeats. Twenty µL of each PCR reaction included a mix of 10 µL qPCR master mix (2X), 8 µL cDNA (4 ng/µL), and 2 µL mix of forward and reverse primers (5 µM). Sequences of the primers used for PCR amplification are shown in Table 1. A 40-cycle, two-step thermal cycling program was set for real-time amplification based on the protocol suggested by the master mix supplier (Yekta Tajhiz Azma Co., Tehran, Iran). According to our previous reports, glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was selected as the best housekeeping gene. The Livak (2-ΔΔCT) method was used for calculating the gene expression data (23).
| Gene | Sequences (5′-3′) | Amplicon Size (bp) |
|---|---|---|
| Gapdh | 77 | |
| F | AGTGCCAGCCTCGTCTCATA | |
| R | GTAACCAGGCGTCCGATAC | |
| Oprm1 | 66 | |
| F | CGATTCCAGAAACCACATTTCA | |
| R | TGTTCGTGTAACCCAAAGCAAT | |
| Drd1 | 58 | |
| F | TCTCCTGGGCAATACCCTTGT | |
| R | GGACCTCAGGTGTCGAAACC | |
| Drd2 | 68 | |
| F | GTCCTGGTACGATGACGATCTG | |
| R | CCTTCCCTTCTGACCCATTG | |
| Cb1r | 78 | |
| F | CCCATTTCAAGCAAGGAGCAC | |
| R | TAGGCCAGACTCAAGGTGACT | |
| Cb2r | 70 | |
| F | CCTTTCCTACTCACTCTGGACA | |
| R | CTACGCCTCTCCTCACTCAG | |
| Tlr4 | 96 | |
| F | CCCTGCCACCATTTACAGTTCG | |
| R | GAGTCCCAGCCAGATGCAAGAG | |
| Tnfα | 140 | |
| F | TGATCGGTCCCAACAAGGA | |
| R | TGCTTGGTGGTTTGCTACGA | |
| Tnfr | 115 | |
| F | TACGGATCCCTCAACCCTGTG | |
| R | CCACAGCATACAGCATCGCAG | |
| Il1β | 74 | |
| F | CAGGATGAGGACCCAAGCAC | |
| R | TCATCCCACGAGTCACAGAGG | |
| Il1r | 95 | |
| F | ACAGGGACTCCTGCTCTGAT | |
| R | TCCCTCTCCGTAGGTCTTGG | |
| Il6 | 72 | |
| F | CCTACCCCAACTTCCAATGCT | |
| R | GGTCTTGGTCCTTAGCCACT | |
| Il6r | 95 | |
| F | CCATCAGGGTCCCATAACAGC | |
| R | TTGCTGTTGTCATTAGGGCAC | |
| Prkcγ | 121 | |
| F | GTATGAGAGAGTGCGGATGG | |
| R | AGTCAGAGATATGCAGGCGTC | |
| Camk2α | 148 | |
| F | GAAGCACCCCAATATCGTC | |
| R | GATACAGTGGCTGGCATCAG | |
| Nos | 140 | |
| F | GAGAGAAAGTACCCGGAACCC | |
| R | GAGACGCTGTTGAATCGGAC | |
| Erk1 | 82 | |
| F | CCAAACAAGCGCATCACAGT | |
| R | CCACTGGTTCATCTGTCGGA | |
| p38α | 104 | |
| F | TGACGAAATGACCGGCTAC | |
| R | AGCCCACGGACCAAATATC | |
| Jnk3 | 132 | |
| F | GCTACAAGGAGAACGTGGAC | |
| R | ACGGAGTTCCTAGCTGCTCTA | |
| Creb | 127 | |
| F | CTGAGGAGCTTGTACCACCG | |
| R | AATCTGTGGCTGGGCTTGAA | |
| NF-kB | 94 | |
| F | TTACGGGAGATGTGAAGATG | |
| R | ATGATGGCTAAGTGTAGGAC | |
| Fos | 74 | |
| F | GGAGCCGGTCAAGAACATTA | |
| R | ATGATGCCGGAAACAAGAAG | |
| Let-7-c1 | 86 | |
| F | GTGCATCCGGGTTGAGGTAG | |
| R | GCTCCAAGGAAAGCTAGAAGGT | |
| mir-124 | 78 | |
| F | CTCTGCTCTCCGTGTTCACA | |
| R | GCTCCGCTCTTGGCATTCA | |
| mir-133 | 73 | |
| F | CTGCTCTGGCTGGTCAAACG | |
| R | CTGCTGTAGCTGGTTGAAGGG | |
| mir-219 | 70 | |
| F | CGGCTCCTGATTGTCCAAACG | |
| R | CGGGACGTCCAGACGCAA | |
| mir-339 | 70 | |
| F | CACCGTCCCTGTCCTCCA | |
| R | TGGACTCTGGCTCTGTCGT | |
| mir-365 | 72 | |
| F | ACAGCAAGAAAAATGAGGGAC | |
| R | GGATTTTTAGGGGCATTATGAC |
Primers Used for Real-time PCR
3.5. Enzyme-Linked Immunosorbent Assay
Triplicate technical repeats were examined for eight biological samples in each experimental group. Ten mg of each tissue sample was homogenized in 100 μL phosphate-buffered saline (PBS) containing protease inhibitor cocktail (Sigma-Aldrich, USA) using a FAPAN 300UPS ultrasonic homogenizer (Fanavari Iranian Pajohesh Nassir, Iran). After centrifugation at 13000 g for 40 minutes, supernatants were transferred to new tubes and stored at -70°C. Enzyme-linked immunosorbent assay (ELISA) kits for proinflammatory cytokines, including interleukin 1-β (IL1-β), tumor necrosis factor-α (TNF-α), and IL6, were used. The manufacturer’s manuals were followed for performing ELISA (DuoSet ELISA kits, R&D Systems, USA). First, a standard curve was plotted for each protein. The concentration of each protein in the examined sample was then measured according to the optical density obtained for each sample by using a microplate reader (BioTek Synergy HTX Microplate Reader, Agilent BioTek, USA).
3.6. Statistical Analysis
First, data were evaluated for normality and equal variance parameters using the Shapiro-Wilk and Brown-Forsythe tests, respectively. Then, between-group comparisons were performed using a two-tailed independent t-test. A probability less than 0.05 (P < 0.05) was set as a significant level throughout the statistical analyses. GraphPad Prism version 9.0 was used for analyzing and plotting data (GraphPad Software, San Diego, California, USA). The corresponding author's data supporting this study's findings are available upon reasonable request.
4. Results
4.1. Frequent Morphine Injection Increased Pro-inflammatory Cytokines at Both Gene and Protein Levels But Downregulated Gene Expression of Their Respective Receptors in the Ventral Striatum
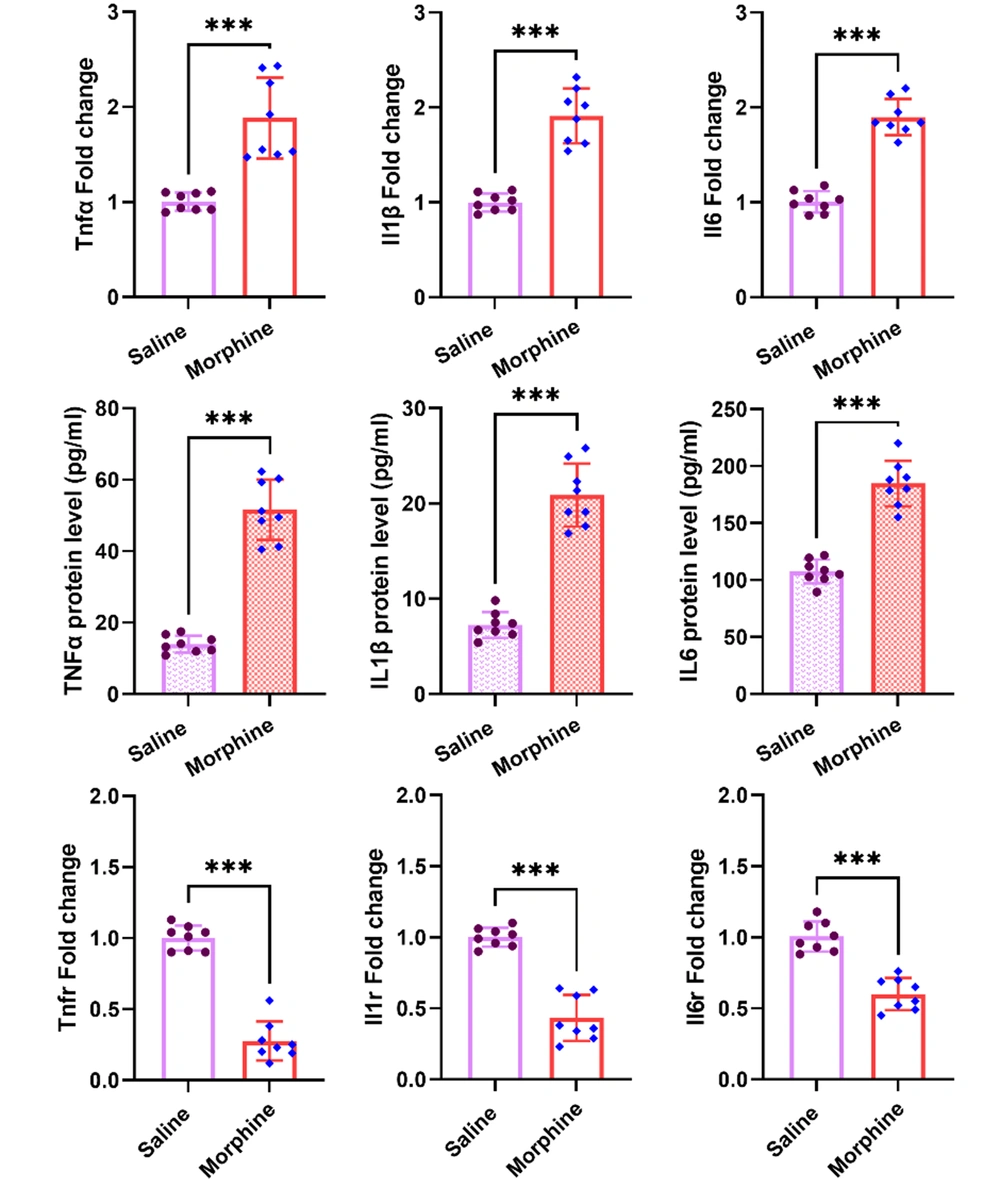
Multiple lines of evidence indicate that morphine induces neuroinflammation, involving in central mechanisms of tolerance and dependence on the drug. We examined the expression of pro-inflammatory cytokines, including TNFα, IL1-β, and IL6 at both gene and protein levels, and gene expression of their respective receptors, including Tnfr, Il1r, and Il6r in the ventral striatum following chronic morphine injections. The qPCR results revealed significant upregulations in mRNA levels of Tnfα [t (14) = 5.7, P < 0.001], Il1 [t (14) = 8.5, P < 0.001], and Il6. Interestingly, there were also significant increases in protein levels of TNFα [t (14) = 12.2, P < 0.001], IL1-β [t (14) = 10.8, P < 0.001], and IL6 [t (14) = 9.7, P < 0.001] in the ventral striatum as revealed by the ELISA results. On the contrary, significant downregulations in mRNA levels of Tnfr [t (14) = 12.6, P < 0.001], Il1r [t (14) = 9.2, P < 0.001], and Il6r [t (14) = 7.3, P < 0.001] were detected in the ventral striatum following chronic morphine treatment (Figure 1).
Effect of frequent morphine injection on the expression of pro-inflammatory cytokines, including TNFα IL1-β, and IL6 at both gene and protein levels, as well as mRNA levels of Tnfr, Il1r, and Il6r in the ventral striatum. The obtained data for each experimental group is reported as the mean ± SD (n = 8). The distribution of the individual data in each group is also seen on each bar. ***: P < 0.001.
4.2. Repeated Morphine Treatment Increased Gene Expression of Oprm1 and Drd1 But Decreased the Tlr4, Cb1r, and Cb2r Expression in the Ventral Striatum
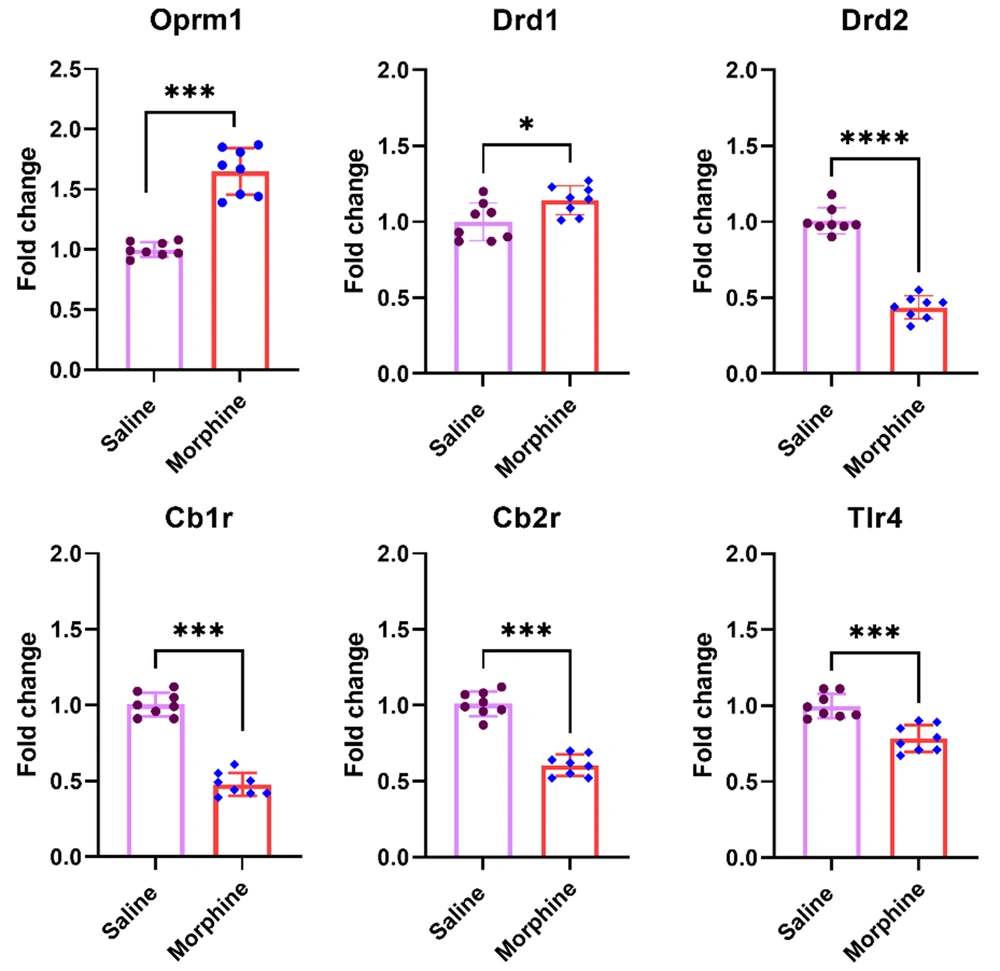
Mu-opioid receptors are the main site of the action of morphine on neurons. In addition, it has also been shown that morphine binds to immune receptors such as TLR4 and activates inflammatory responses. In the second experiment of the present study, mRNA levels of Oprm1, Drd1 and Drd2, Tlr4, Cb1r, and Cbr2 were evaluated in the ventral striatum following chronic morphine treatment. The results of this experiment revealed significant increases in gene expressions of Oprm1 [t (14) = 9, P < 0.001] and Drd1 [t (14) = 2.6, P < 0.05] but the expression of Drd2 [t (14) = 14.08, P < 0.001], Tlr4 [t (14) = 5.08, P < 0.001], Cb1r [t (14) = 13.8, P < 0.001], and Cb2r [t (14) = 10.7, P < 0.001] significantly decreased in the ventral striatum in rats following chronic morphine injection compared to the group treated with saline (Figure 2).
Effect of chronic morphine injection on gene expression of Oprm1, Drd1, Drd2, Tlr4, Cb1r, and Cb2r in the ventral striatum. The mRNA level in each experimental group is reported as the mean ± SD (n = 8). The distribution of the individual data is also seen on each bar. *: P < 0.05 and ***: P < 0.001.
4.3. Repeated Morphine Injection Decreased mRNA Levels of Protein Kinase Cγ, Calcium/Calmodulin-Dependent Kinase IIα, and Nitric Oxide Synthase, as Well as p38α and Jnk3 MAP Kinases in the Ventral Striatum
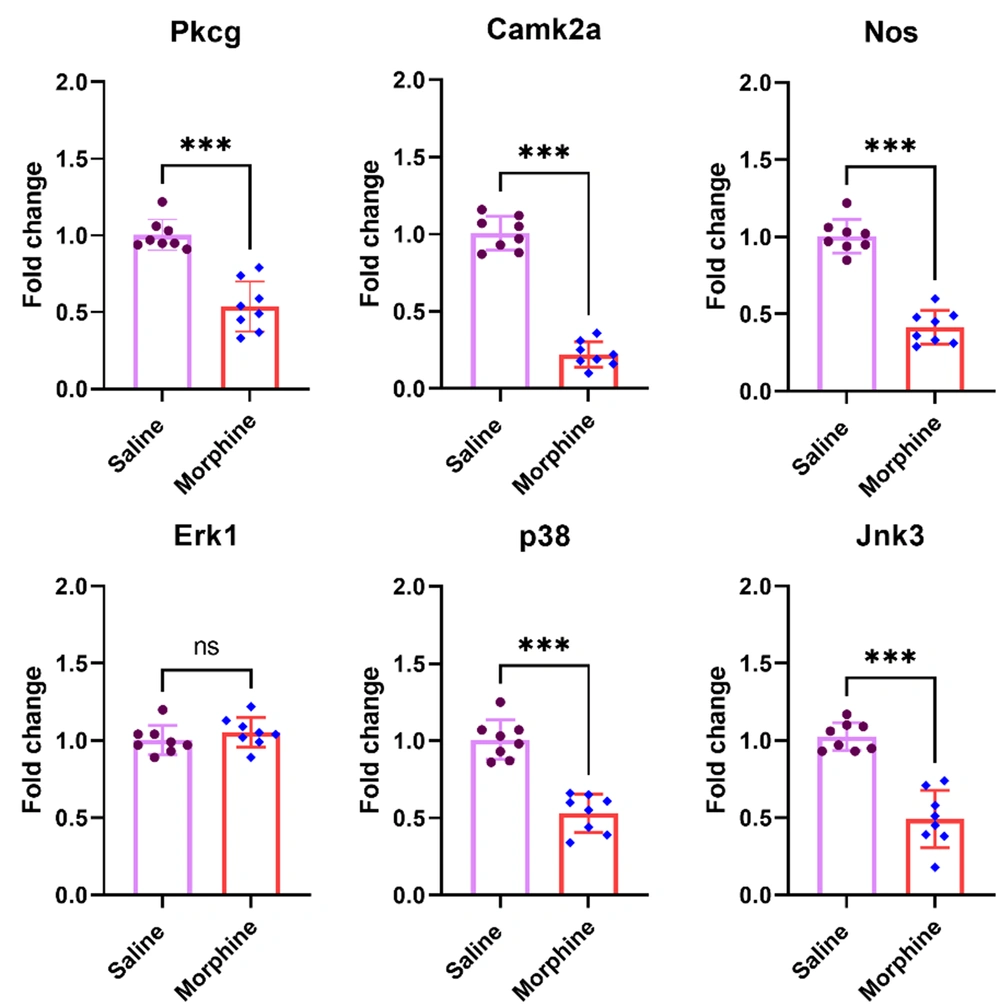
Different kinases modulate the cellular adaptations connected with opioid tolerance and dependence (24). We examined mRNA levels of Prkcγ, Camk2a, and Nos, and three members of MAP kinases, including, Erk1, p38, and Jnk3, in the ventral striatum after chronic administration of morphine. The results showed significant decreases in the gene expression of Prkcγ [t (14) = 6.9, P < 0.001], Camk2a [t (14) = 16.1, P < 0.001], Nos [t (14) = 10.9, P < 0.001], p38 [t (14) = 7.6, P < 0.001], and Jnk3 [t (14) = 7.3, P < 0.001] in the ventral striatum in rats receiving chronic morphine compared to the control group. However, there was no group difference between the experimental groups in the Erk1 expression [t (14) = 1.04, P = 0.315] (Figure 3).
Effect of chronic morphine injection on the expression of Prkcγ, Camk2a, Nos, Erk1, p38, and Jnk3 MAP kinases in the ventral striatum. The mRNA level in each experimental group is reported as the mean ± SD (n = 8). The distribution of the individual data is also seen on each bar. ns, non-significant and ***: P < 0.001.
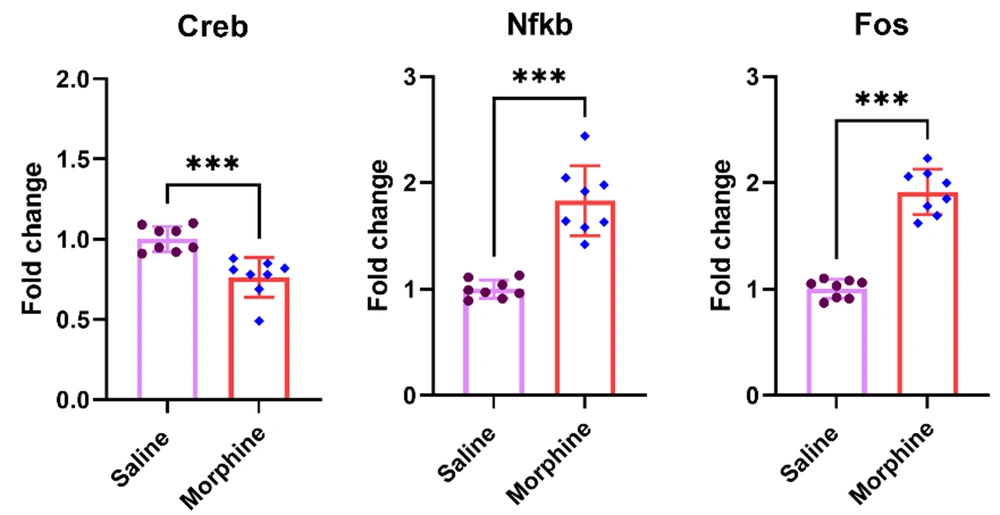
4.4. Chronic Morphine Injection Decreased Creb But Increased Nfkb and Fos mRNA Levels in the Ventral Striatum
Transcription factors mediate the chronic impacts of morphine on gene expression (1, 25). The results of the present experiment revealed that chronic morphine injection significantly decreased the Creb gene expression [t (14) = 4.64, P < 0.001] but significantly increased the expression of Nfkb [t (14) = 6.9, P < 0.001] and Fos [t (14) = 11.2, P < 0.001] in the ventral striatum compared to the control group treated with saline (Figure 4).
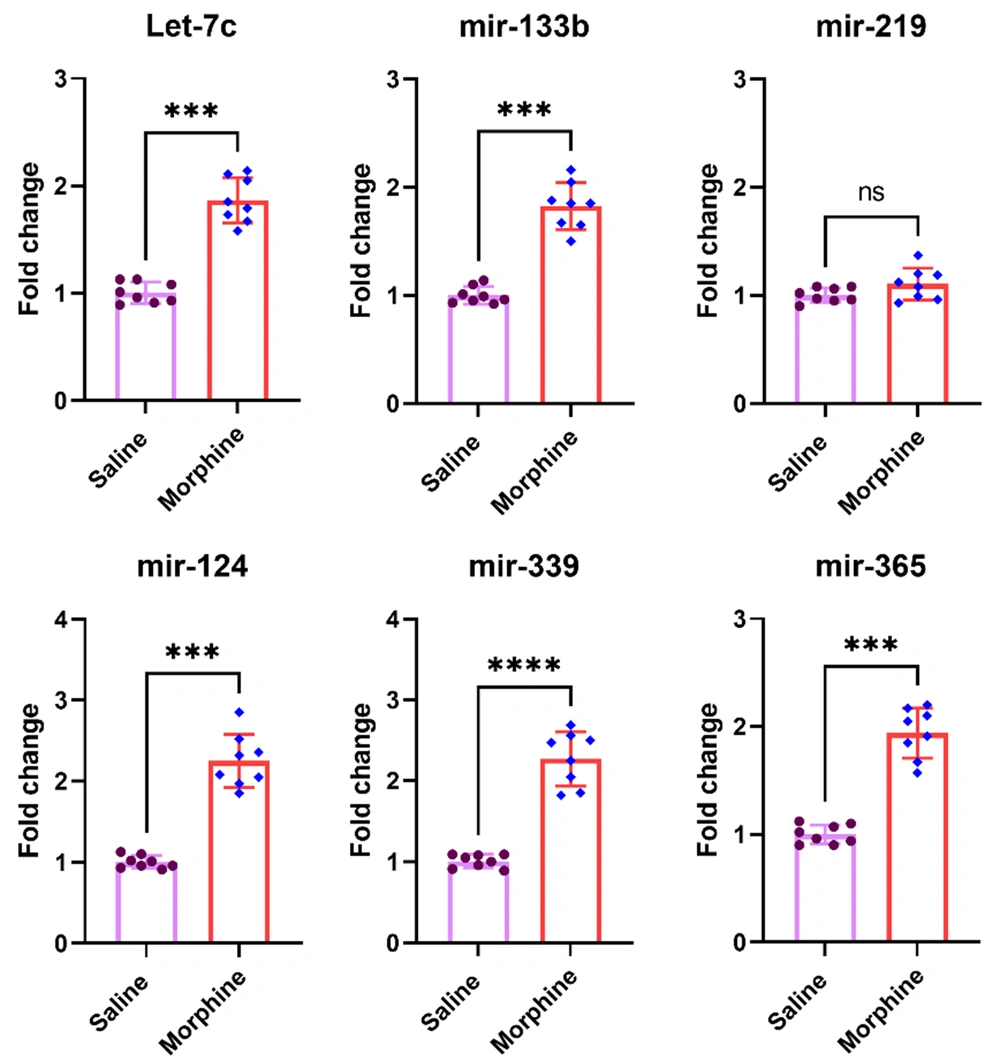
4.5. Expression of Biologically-Relevant miRNAs to Morphine Addiction is Upregulated in the Ventral Striatum After Chronic Morphine Treatment
Six miRNAs, including Let-7c1, miR-133b, miR-219, miR-124, miR-339, and miR-365, had been reported by different investigators to be affected in different brain reward areas related to addiction (17, 26). The results of the current study revealed that chronic morphine treatment significantly increased expression of let7c1 [t (14) = 10.4, P < 0.001], mir-133b [t (14) = 10.2, P < 0.001], mir-124 [t (14) = 10.4, P < 0.001], mir-339 [t (14) = 10.4, P < 0.001], and mir-365 [t (14) = 10.7, P < 0.01] in the ventral striatum. However, no significant group difference was detected for mir-219 expression [t (14) = 1.8, P = 0.095] in the ventral striatum after frequent morphine treatment (Figure 5).
Effect of chronic morphine injections on the expression of let7c1, mir-124, mir-133b, mir-219, mir-339, and mir-365 in the striatum. The pre-miRNA level in each experimental group is reported as the mean ± SD (n = 8). The distribution of the individual data is also seen on each bar. ns, non-significant and ***: P < 0.001.
5. Discussion
Accumulating evidence shows that long-term use of opiates reorganizes the cellular and molecular machinery, especially in the brain regions associated with reward (27, 28). It has been postulated that alterations in gene expression following prolonged use of morphine initiate structural and functional remodeling in the neural pathways, leading to tolerance to the rewarding and analgesic effects of the drug and dependence (29, 30). We have shown in our previous reports that frequent administration of morphine for eight successive days induces tolerance to the analgesic effect of morphine and drug dependence in rats (31, 32).
The present results demonstrated that frequent exposure to morphine notably increased the expression of pro-inflammatory cytokines in the ventral striatum. It has also been shown that opioid binding to TLR4 leads to the activation of NF-κB, increasing pro-inflammatory cytokines, including TNFα, IL-1β, and IL-6 (15). NF-κB regulates opioids and pro-inflammatory cytokines gene expression in neuronal and immune cells, affecting opioid-induced biological responses (33). Therefore, significant increases in the Nfkb mRNA level in the present experiment may finally mediate upregulations in pro-inflammatory cytokines following frequent morphine exposure in the ventral striatum. Further, FOS is a subunit of the activator protein-1 (AP-1) that drives transcription from different genes, including inflammatory cytokines (34, 35). Alteration in Fos expression in striatal neurons caused long-term changes in gene expression, mediating changes in brain circuits (36). There is a reciprocal interaction between IL-1β, TNFα, and c-Fos/AP-1 influencing activity and gene expression resulting in joint destruction in rheumatoid arthritis (37). NF-κB and AP-1 also have reciprocal modulating effects on each other, affecting the transcription of inflammatory cytokines and other genes (38). Taken together, chronic morphine treatment via affecting inflammatory cytokines and transcription factors orchestrate transcriptional machinery, leading to changes in striatal circuits. In addition, the significant downregulations of Il1r, Il6r, and Tnfr may reveal a homeostatic adaptation to the significant increases in pro-inflammatory cytokines and subsequent overactivation of the respective receptors. Our results support the idea of using cytokine-sequestration peptides as a potential adjunct to opioid therapy, as proposed by some investigators (39).
It has been shown that long-term administration of morphine induces desensitization and/or endocytosis of MORs to attenuate input signaling to the cell (40). However, the increased Oprm1 mRNA due to frequent morphine exposure in the present study may indicate a compensatory mechanism in response to the possible decreases in MORs in the ventral striatum (41). Further, accumulating evidence also shows that morphine, via binding to TLR4, increases pro-inflammatory cytokines, which partly mediate opioid tolerance and dependence (6, 42). Edison and Murphy reported that the pharmacological antagonism of TLR4 in the periaqueductal gray matter diminished morphine-induced analgesic tolerance and increased the antinociceptive properties of the drug (4). Therefore, a decreased level of Tlr4 mRNA may reflect a homeostatic response to the frequent use of the opioid and its binding to TLR4 in the ventral striatum.
Medium-sized spiny neurons (MSNs) in the ventral striatum express DRD1 and DRD2, which are activated by phasic and tonic increases in dopamine levels, respectively (43). There is some evidence that simultaneous activation of DRD1 and DRD2 in the ventral striatum following an increase in dopamine level leads to maximal reward and reinforcement (44). The tonic dopamine release in the ventral striatum acts mainly on MSNs via DRD2 (3). A possible explanation for the present results is that repeated exposure to morphine increases dopamine release more slowly with a tonic pattern in the ventral striatum, causing sustained activation of DRD2 and decreasing the Drd2 gene expression. On the contrary, activation of DRD1 in the ventral striatum needs a phasic increase in dopamine level following a burst firing of dopaminergic afferents (3). Therefore, an increase in Drd1 mRNA level after chronic morphine exposure may reveal a reduction in the phasic release of dopamine after frequent morphine injections.
Cannabinoid CB1 receptors are co-expressed with MORs in the ventral striatum (45). Both cannabinoid receptors, including CB1R and CB2R, are significantly involved in drug reward and addiction (46, 47). Deleting the Cb1r gene in mice has revealed a major influence on MORs accessibility on the dopamine innervation of the ventral striatum (48). Alterations in Cb1r and Cb2r gene expression in the ventral striatum following frequent morphine injection further support the involvement of cannabinoid receptors in morphine actions and improve this idea that morphine either through MORs and the downstream intracellular signaling cascade or via increases in pro-inflammatory cytokines in the ventral striatum affects gene expression of the cannabinoid receptors. Taken together, based on mRNA levels of Oprm1, Tlr4, Drd2, Cb1r, and Cb2r genes detected in the present study and considering the above-cited reports, we propose that increases in pro-inflammatory cytokines in the ventral striatum due to frequent morphine treatment finally tend to alter the molecular machinery and the responsiveness of the reward pathways to long-term opioid treatment.
Different protein kinases are involved in neuroadaptive changes in intracellular signaling pathways following chronic morphine treatment. In particular, both protein kinase C (PKC) and calcium/calmodulin-dependent protein kinase II (CaMKII) are involved in the induction of tolerance after chronic morphine treatment (24). Prolonged exposure to morphine via overactivation of adenylate cyclase and a CREB pathway increases the expression of PKCγ and N-Methyl-D-Aspartate (NMDA) receptors in the spinal cord (49, 50). Long-term use of morphine impairs cognitive functions (51, 52), and it has been shown that nitric oxide (NO) has a key role in mediating the adverse effects of morphine on cognitive functions (53). Chronic morphine treatment decreased iNos expression in the hippocampus, leading to decreased cognitive performance (54). Therefore, decreases in the expression of Prkcγ, Camk2a, and Nos in the ventral striatum may finally impair cognitive functions following chronic morphine treatment. Further, the CREB family of transcription factors has a key role in memory formation, and their inhibition causes memory impairment (55). Therefore, we propose that downregulations of Creb and upstream signaling molecules in the ventral striatum in response to chronic morphine exposure have a role in memory impairment induced by opioids.
The results of the present experiments revealed that chronic morphine exposure, increased the expression of Let-7c1, mir-124, mir-133b, mir-339, and mir-365 but not mir-219 in the ventral striatum. Morphine significantly upregulated let-7 miRNA expression in a mouse model of opioid tolerance, which was antagonized by decreasing Let-7 miRNA levels by an inhibitor (56). Based on target prediction for Let-7c1 mature miRNAs in the TargetScan database, we found that Let-7c1 could target Il6r, Tlr4, Creb, and Jnk3 transcripts, suggesting a possible role for Let-7c1 in mediating the decreases in those genes in the ventral striatum. In addition, mature miRNAs derived from mir-124 have the potentials to target Creb, p38α, and Jnk3 transcripts, and the increased level of mir-124 may account for the decreases in these genes in the ventral striatum. Morphine treatment increased miR-339-3p and decreased MOR expression in the hippocampus in mice (57). However, the increased level of mir-339 in the ventral striatum did not decrease Oprm1 gene expression, suggesting complex mechanisms for controlling Oprm1 gene expression in different brain regions. Wang et al. reported that overexpression of miR-365 through decreasing β-arrestin2 protein in the spinal cord prevents morphine tolerance (26). Further, Wu et al. reported that miR-365 decreased morphine analgesic tolerance by targeting β-arrestin2 and inhibiting the activation of the ERK/CREB signaling pathway (58). However, mir-365 significantly increased in the ventral striatum in rats receiving frequent morphine injections. Based on the target prediction evaluations, mir-365 may be involved in the downregulation of Drd2, Tlr4, Il1r, Il6r, and Tnfr gene transcripts. To the best of our knowledge, the present results for the first-time show increases in the examined miRNAs and their possible association with downregulations in the above-mentioned genes in the ventral striatum after chronic morphine exposure in rats. However, a limitation of the current study was lacking protein levels for some of the examined genes to thoroughly investigate their functional role in neuroadaptive changes in the ventral striatum after long-term use of morphine. Therefore, further studies are needed to investigate the functional consequences of the alterations at the mRNA levels in the ventral striatum in response to chronic morphine exposure.
5.1. Conclusions
The current results revealed that chronic morphine exposure caused significant increases in pro-inflammatory cytokines at gene and protein levels in the ventral striatum. Notable increases in the expression of Oprm1, Drd1, Fos, and Nfkb at the mRNA level, as well as expression of specific miRNAs in the ventral striatum, were detected following chronic morphine treatment. Significant downregulations were also detected in Tlr4, Il1r, Il6r, Tnfr, different kinases, and Creb in the ventral striatum following frequent morphine treatment, suggesting that the dysregulations in pro-inflammatory cytokines and the downstream signaling pathways impair physiological functions of the ventral striatum following chronic morphine exposure. Changes at mRNA levels of the examined genes after chronic morphine injections reveal highly specialized roles with possible functional meaning in the induction of neuroinflammation as well as establishing new setpoints in the ventral striatum, affecting the behavioral expressions related to morphine tolerance, dependence, and addiction. Further, miRNAs, especially Let-7-C1, mir-124, and mir-365, play key roles in mediating chronic morphine effects on post-transcriptional levels of the cytokine receptors and downstream signaling pathways, which needs further consideration.