1. Background
Colon cancer is a common type of cancer worldwide. Despite standard treatments, its mortality is high due to drug resistance (1). Therefore, exploring the new anticancer drugs and the involved mechanisms may help to manage this cancer. Mesenchymal stem cells (MSCs) are non-hematopoietic and adherent cells that differentiate into different cell types (2). Furthermore, MSCs secrete soluble factors with various paracrine functions that affect cellular processes such as angiogenesis, apoptosis, and immune response into the microenvironments (3, 4). Adipose tissue, placenta, umbilical cord, and bone marrow are the main sources of MSCs (5).
Adipose tissue is a rich source of stem cells with fewer complications for patients. Furthermore, a small volume of fat tissue is required to isolate MSCs compared to the other origins (6). Adipose-derived mesenchymal stem cells (ASMCs) release a complex of cytokines and growth factors into the extracellular microenvironment. The combination of these factors is named secretome. The secretome plays a dual role in regulatory mechanisms and can either inhibit or promote the proliferation of malignant cells (7, 8). Several studies have explored the effect of MSCs on various malignant cells or tissues (9, 10). However, their obtained results are controversial. The controversial results may be due to various MSC sources or cancer types (11). Mesenchymal stem cells prevent the growth of some malignancies, including colon cancer, hepatoma, and melanoma (12-14).
2. Objectives
In the current work, the cytotoxic and apoptotic impacts of adipose mesenchymal stem cells (AMSCs)-adipose mesenchymal stem cells-derived secretome (AMSC-Se) against a colon cancer cell line were explored.
3. Methods
3.1. Adipose Mesenchymal Stem Cell Characterization
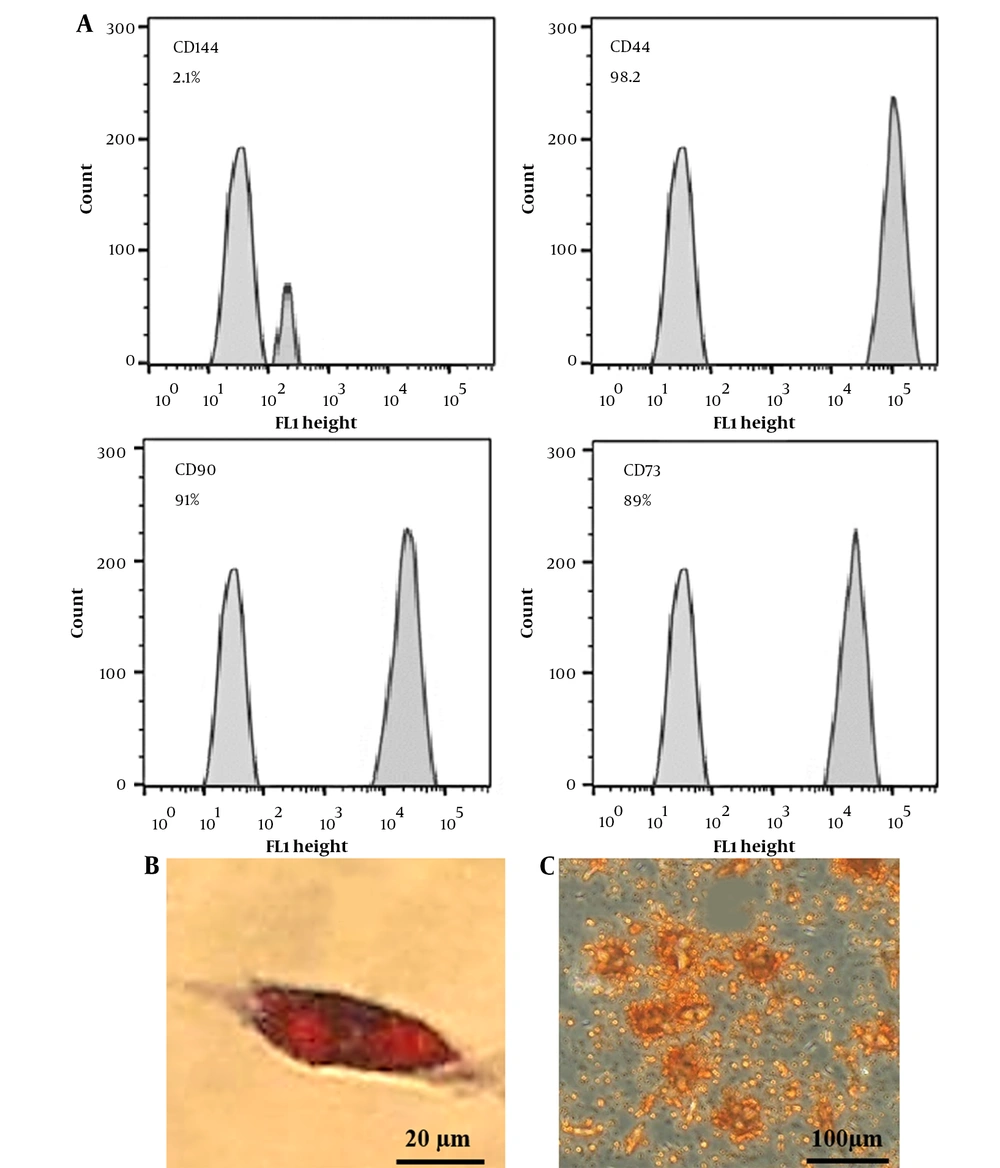
The human AMSCs were from Royan Company, Tehran, Iran. The cells have been characterized (in passage 3) using flow cytometry to determine specific markers. Adipogenic and osteogenic potentials were evaluated by oil-red and Alizarin-Red staining (Figure 1).
Characterization of AMSCs by flow cytometry (A). AMSCs were found to be positive for MSC-specific markers (CD90, D73, and CD44), and negative for hematopoietic stem cell surface markers (CD144). Adipogenic and osteogenic differentiation potential of AMSCs (B and C). Oil red O (B) and Alizarin red staining (C) positive cells, showing adipogenic and osteogenic differentiation of the AMSCs.
3.2. Secretome Preparation
Briefly, the AMSCs were harvested in complete media. At 80% confluency, the cells were washed and incubated in a serum-free DMEM overnight. The conditioned media were centrifuged at 4,000 g for 10 min. Then, the conditioned media were re-centrifuged at 4,000 g with an Amicon Ultra-15 centrifugal filter (Sigma) for two hours (15). The protein contents were determined by a BCA kit (Invitrogen, USA) and stored at -80°C.
3.3. Cell Culture
The HT-29 cells (RRID: CVCL_0320) were cultured in McCoy's 5a media with two mM glutamine and FBS (10%) and incubated in the cell culture incubator. At 80% confluency, the media had discarded, and the cells were treated with 0 (control), 50, and 100 µg/mL of AMSC-Se. The optimum concentration and exposure time of AMSC-Se were obtained from the MTT results (Table 1).
3.4. Cell Viability
The 106 cells were harvested in 96-well dishes for one day. The cells exposed to AMSC-Se and 0.5 mg/mL of MTT solution were added to the wells and kept for three hours in an incubator. The supernatants were removed, 100 µL DMSO was added, and the absorbance was read at 570 nm using a BioRad microplate reader. The percentage of cell viability was calculated relative to the control cells.
3.5. Real-time PCR
The RNA was extracted from the cells (107 cells) using an RNeasy kit (Qiagen, Valencia, USA). The quality of extracted RNA was determined by measuring the UV absorption of the samples. A cDNA synthesis kit (Qiagen) was used to synthesize cDNA. The cDNAs were amplified in the PCR reaction solution containing SYBR green master mix and primers. The primer sequences were: Bcl-2: Forward 5′-GGATGCCTTTGTGGAACTGT-3′, Reverse 5′-TCACTTGTGGCCCAGATAGG-3′; Bax: Forward 5′-GCTGGACATTGGACTTCCTC-3′, Reverse 5′-ACCACTGTGACCTGCTCCA-3′ and GAPDH: Forward 5′-GCAAGAGCACAAGAGGAAGA-3′, Reverse 5′-ACTGTGAGGAGGGGAGATTC-3′.
A 45-cycle program was considered for PCR amplification: initial denaturation (95°C, 10 seconds), denaturation (95°C, 15 seconds), annealing (55°C, 20 seconds), and extension (60°C, 20 seconds).
3.6. Measuring Protein Level of Bax and Bcl-2
The cultured cells were mixed with RIPA lysis buffer plus protease inhibitor. BCA assay kit (Sigma, USA) was used to obtain protein from the cells. The protein level of the Bax and Bcl-2 were measured using ELISA kits (R&D).
3.7. Caspase Activity Assay
The caspase activities were measured by Caspase Assay Kits (Thermo Fisher, USA). The 106 cells were exposed to 50 or 100 µg/mL AMSC-Se for one day. Then, cell lysates were provided and centrifuged at 17,000 rpm for 15 min. The amount of protein was determined by a BCA assay Kit. The cell lysates were exposed to caspase substrate for four hours. The absorbance was read at 405 nm by a Bio-Rad microplate reader.
3.8. Statistical Analysis
Data were analyzed with one-way ANOVA followed by Tukey's post hoc test for multiple pairwise comparisons. The P-values < 0.05 were considered significant.
4. Results
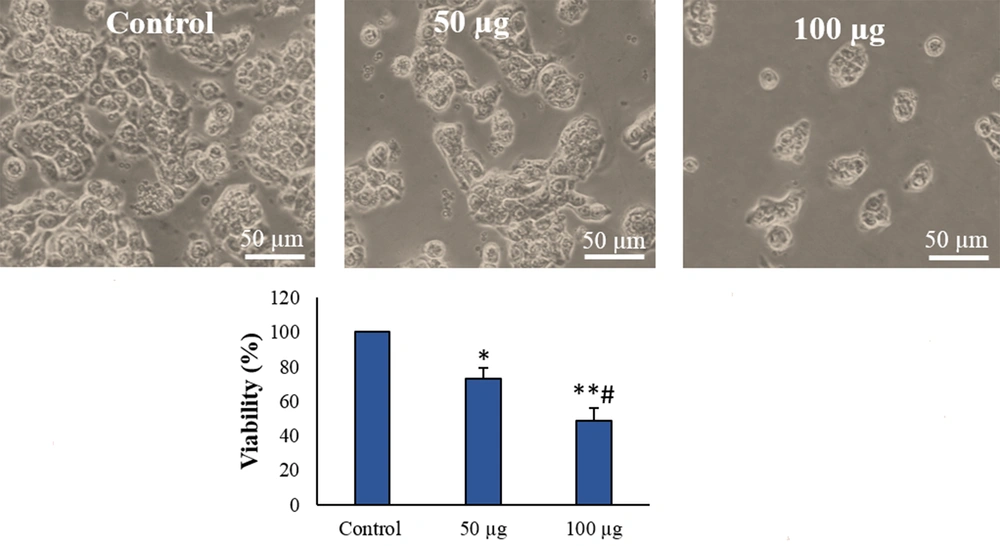
4.1. Adipose Mesenchymal Stem Cells-Derived Secretome Exposure Inhibited the Cell Growth
Adipose mesenchymal stem cells-derived secretome decreased the survival of the cells concentration-dependently. The survival rate (Figure 2) decreased in the 50 μg/mL AMSC-Se-exposed cells compared to the control (P < 0.05). The survival rate of the 100 μg/mL AMSC-Se-exposed cells was reduced compared to the untreated and 50 μg/mL-treated cells (P < 0.05).
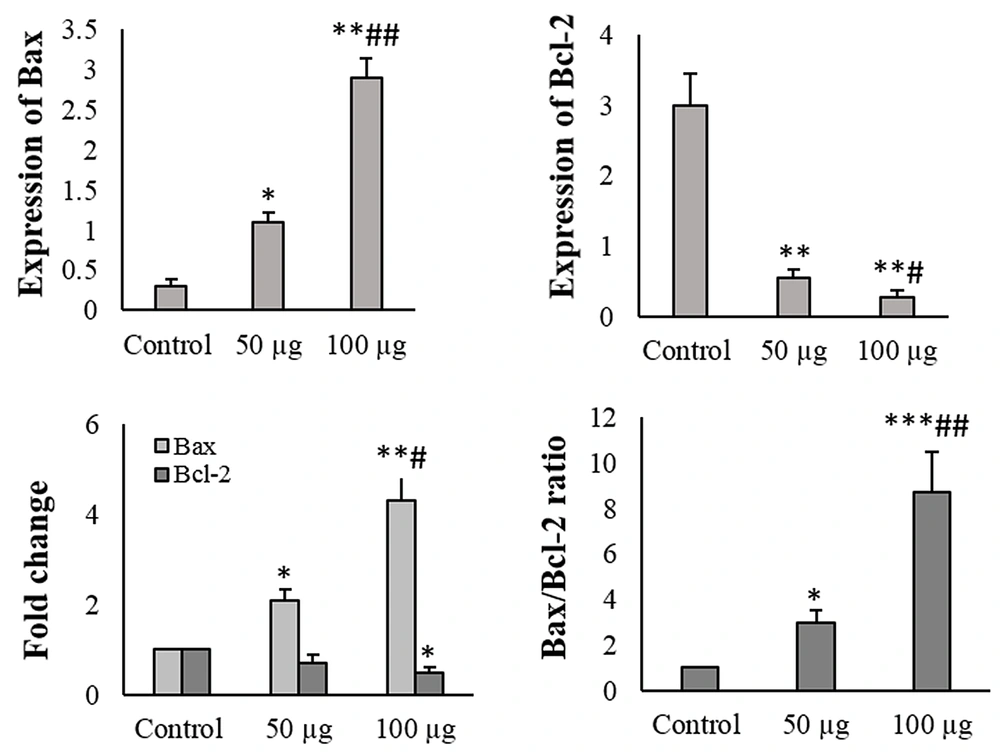
4.2. mRNA and Protein Expression of Apoptosis-Related Proteins
Adipose mesenchymal stem cells-derived secretome changed the mRNA and protein expression of Bcl-2 and Bax in the HT-29 cells concentration-dependently. Adipose mesenchymal stem cells-derived secretome at the 50 μg/mL concentration enhanced the mRNA and protein level of Bax (P < 0.05), while the Bcl-2 expression was diminished compared to the control (P < 0.05). Adipose mesenchymal stem cells-derived secretome at 100 μg/mL elevated the mRNA and protein level of Bax and decreased Bcl-2 expression compared to the untreated and 50 μg/mL AMSC-Se-exposed cells (Figure 3).
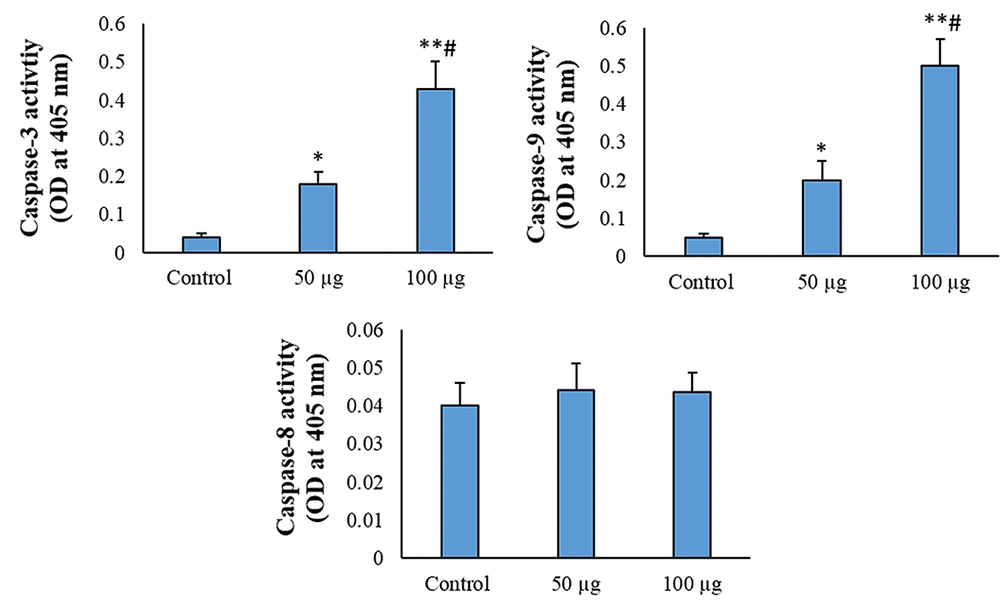
4.3. Caspase-3 and Caspase-9 Are Involved in the AMSCs-Se-Induced Apoptosis
The caspase-3 and caspase-9 activities enhanced in the 50 μg/mL AMSC-Se group in comparison to the control (P < 0.01). The activities of caspase-3 and caspase-9 were also significantly enhanced in 100 μg/mL AMSCs-Se (P < 0.001) and 50 μg/mL (P < 0.05) treatments compared to the control. The caspase-8 activity did not change by the AMSCs-Se (Figure 4).
5. Discussion
In this work, the effect of AMSC-Se on HT-29 cell growth has been explored. The results indicated that AMSC-Se significantly diminished proliferation and enhanced apoptosis of HT-29 cells. In line with these results, Zhu et al. revealed that the factors secreted by AMSCs could suppress the survival and proliferation of the K562 leukemia cells (16). Also, Ryu et al. reported that AMSCs conditioned medium prevents breast cancer cell growth (17). Adipose mesenchymal stem cells suppressed the growth of SKBR3 (a breast malignant cell line) cells in direct co-cultures (18). In another investigation, AMSC-Se reduced bladder cancer cell viability and decreased its resistance to ciprofloxacin (7).
In contrast, some reports have demonstrated that secretome from AMSCs promotes the proliferation of breast cancer cells (19, 20). Conditioned medium from AMSCs significantly enhanced hepatocellular carcinoma invasion (21). In another study, AMSC-Se promoted the growth of prostate cancer cells (22). These controversial results may be due to the variability of MSC sources or cancer cell types.
Flow cytometry method was used in this study to explore the relationship of anti-proliferating impact of AMSC-Se with apoptosis. The AMSC-Se-treated cells exhibited more apoptosis than the untreated cancerous cells. Previous studies showed that conditioned medium from AMSCs induces apoptosis in the cancerous cells (23, 24). In this regard, AMSCs increased apoptosis along with proliferation inhibition in hepatocellular carcinoma cell lines (25). Direct co-culture of SKBR3 cancer cells with AMSCs increased chemosensitivity and enhanced apoptosis in response to 5-fluorouracil and doxorubicin (18). Adipose mesenchymal stem cells-derived secretome also prevented melanoma proliferation by inducing apoptosis in vitro (24). As shown in flow cytometry results, the necrosis percentage did not change by the AMSC-Se, which revealed a critical role of apoptosis in reducing the survival of the malignant cells of the AMSC-Se.
Protein and mRNA levels of Bcl-2 and Bax, and caspases activity were assessed in the current work to confirm the involvement of apoptosis in AMSC-Se-reduced growth of the cancer cells. Adipose mesenchymal stem cells-derived secretome enhanced the Bax level, while the Bcl-2 level was reduced within the HT-29 cells. These genes are the main regulators of the intrinsic apoptotic pathway. The enhanced Bax/Bcl-2 ratio expression activates caspase-3 and caspase-9 via cytochrome-c release and eventually induces apoptosis (25). Our results demonstrated the treatment AMSC-Se considerably enhanced the activity of caspase-3 and caspase-9. The activated caspase-9 can activate effector caspases and initiate the intrinsic apoptotic pathway.
Conditioned media from human AMSCs could elevate the activities of caspase-3 and caspase-9 and lead to apoptosis in the human U251 glioma cells (26). Therefore, these results suggest that AMSC-Se stimulates the intrinsic pathway of apoptotic to enhance cancer cell death. The secretome of the MSCs has biological components, such as cytokines, growth factors, angiogenic factors, micro RNAs, anticancer molecules, and exosomes (27-34). A number of studies evaluated the effects of the secreted molecules from the MSCs. Bone marrow MSCs-derived cytokine-induced neutrophil chemoattractant-1 and tissue inhibitor of metalloproteinases-1 could prevent the proliferation of the myeloid leukemia cells by suppressing the caspase-3 pathway (35). TRAIL can initiate the caspase-8 cascade and promote the extrinsic apoptotic pathway (36).
5.1. Conclusions
The present study has revealed the cytotoxic impacts of AMSC-Se on the HT-29 cells. These findings clearly indicated that the inhibitory effect of AMSC-Se against HT-29 cell growth is modulated by the stimulating intrinsic apoptotic signaling pathway.