1. Context
Alzheimer's disease (AD) is a neurodegenerative condition associated with aging, affecting more than 25 million people worldwide. It is characterized by cognitive changes, memory deficits, and behavioral alterations, making it a leading cause of dementia among the elderly. AD can be categorized based on the age of onset into sporadic cases (which constitute the majority of AD instances) and familial cases (comprising 5 to 10% of patients) with an autosomal dominant inheritance pattern (1).
The pathophysiology of AD is marked by synaptic injury, neuronal loss, astrogliosis, the presence of senile plaques containing amyloid-β (Aβ) peptides, hyperphosphorylation of tau protein, and the development of neurofibrillary tangles (2). Recent studies (3, 4) have demonstrated that therapies targeting Aβ have not been successful in treating AD, challenging the prevailing amyloid-tau theories regarding disease causation. In reality, Aβ and tau (misfolded proteins) accumulate within neurons as a consequence of the disease, and they may be released into extracellular spaces following neuronal death (5).
The observed decline in melatonin levels in bodily fluids and reduced expression of MT1 and MT2 receptors in AD-afflicted brains suggest a significant connection between the melatonin system and the disease (6, 7).
The versatile hormone melatonin possesses various beneficial properties, including anti-inflammatory, cytoprotective, and antioxidant effects, in addition to its role in regulating the circadian rhythm. Melatonin production is subject to circadian regulation, with research conducted in mouse and rat models revealing that peak plasma melatonin levels typically occur around midnight (8). The aging process leads to a decline in melatonin synthesis, a factor that may play a crucial role in the development of AD (9).
During AD, several alterations have been observed in the hormone melatonin (MLT) produced by the pineal gland, as well as in the activity of enzymes involved in MLT synthesis and the density of MT1 receptors in the suprachiasmatic nucleus (SCN) (10). When comparing AD patients to healthy individuals, it becomes evident that melatonin levels are lower in the former group. Melatonin's ability to scavenge free radicals and its anti-amyloidogenic effects make it a promising candidate for slowing the progression of AD. Additionally, melatonin prevents the secretion of soluble amyloid precursor protein (APP) by promoting APP maturation in various cell types. Both in vitro and in vivo studies have shown that melatonin treatment reduces the production and deposition of Aβ (11).
In a study conducted by Lee et al., it was discovered that melatonin has the ability to reverse memory impairment induced by stress in rats. This reversal occurs through the action of cAMP-response element-binding (CREB) protein in the hippocampus and the inhibition of proinflammatory cytokines in the brain, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (12). These findings highlight melatonin's anti-inflammatory properties. Additionally, several other animal studies have shown that melatonin (MLT) could effectively mitigate learning and memory deficits in cases of vascular dementia (13).
The hyperphosphorylation of tau protein in AD has been extensively documented. Research has demonstrated that melatonin, by modulating the activity of enzymes involved in tau phosphorylation, specifically glycogen synthase kinase 3β (GSK3β) and cyclin-dependent kinase 5 (Cdk5), can reduce the extent of hyperphosphorylation (14).
Tau protein acetylation also plays a role in AD progression, leading to its abnormal aggregation. Acetylation at specific residues disrupts tau's normal sorting and promotes aggregation by interfering with ubiquitin-mediated clearance. Melatonin has been shown to increase the expression of the sirtuin 1 gene (SIRT1), which is a deacetylase (15). In transgenic mouse models of AD, SIRT1 has been found to reduce the amyloidogenic processing of βAPP (16).
Hence, it appears that melatonin (MLT) may exert a protective effect against AD by inducing the overexpression of SIRT1. A substantial and growing body of literature has focused on the potential of MLT as an intervention for AD. Recent studies have indicated that MLT could potentially serve as a marker for assessing the severity and progression of AD. This paper aims to review recent research in three main areas: (1) MLT physiology, (2) the role of MLT in learning and memory processes, and (3) discussions on studies investigating the role of MLT in AD.
2. Melatonin and Its Potential Targets
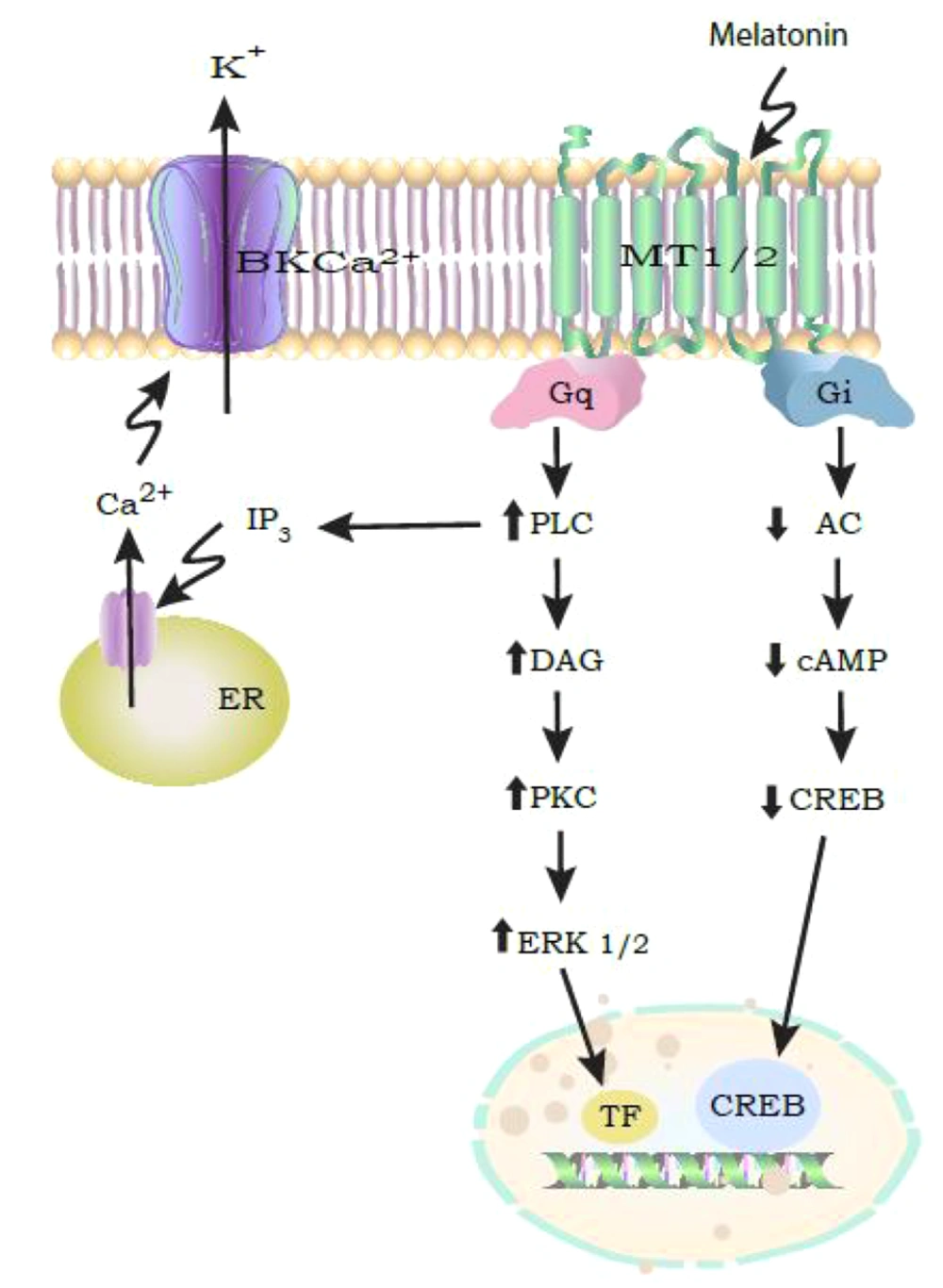
MLT functions by binding to MT1 and MT2 receptors located in the plasma membrane. These receptors are part of the G-protein coupled receptors (GPCR) subfamily and are expressed in various brain regions and peripheral tissues (17). In humans, MT1 receptors are found in the frontal, temporal, parietal, and occipital cortex, thalamus, hippocampus, and supra-optic nucleus (SCN). MT2 receptors are expressed in the cerebellum, hippocampus, and SCN. MLT receptors play a role in modulating diverse physiological functions, including circadian rhythms, sleep, and learning and memory processes. The MT1 and MT2 receptors are primarily coupled to Gi/o proteins, leading to reduced cAMP levels and the inhibition of protein kinase A (PKA). Additionally, MLT can regulate ion channels, such as large-conductance Ca2+-activated K+ channels, through pathways involving Gq/PLC/Ca2+ or Gi/cAMP/PKA (Figure 1) (18).
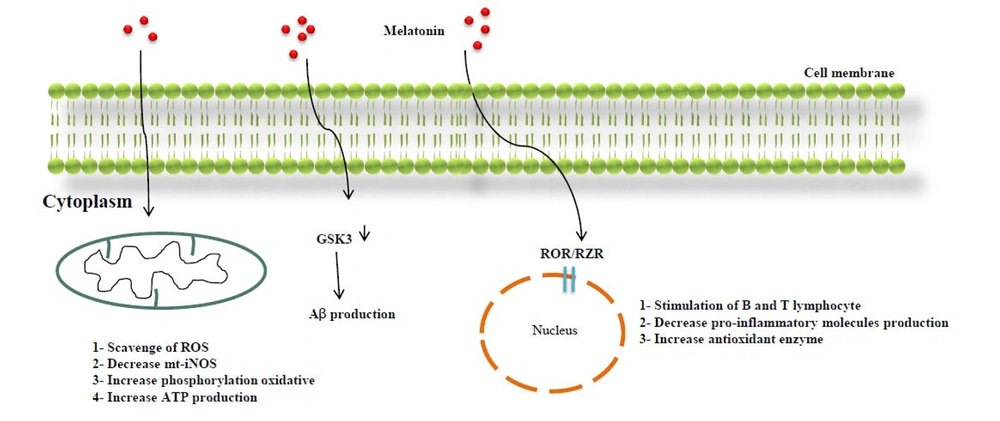
The MT3 receptor corresponds to a cytosolic enzyme called quinone reductase 2 (QR2), which is primarily found in peripheral organs, such as the liver, kidney, heart, and lungs (18, 19). MLT possesses a lipophilic property, allowing it to permeate cell membranes directly through simple diffusion (20). MLT's entry into cells has several effects: It scavenges reactive oxygen species (ROS) and reduces inducible nitric oxide synthase (m-iNOS) expression, thus promoting oxidative phosphorylation and ATP production within mitochondria. Additionally, MLT binding to the nuclear retinoid Z receptor (ROR/RZR) protein family stimulates A and B lymphocytes, reduces the production of pro-inflammatory molecules, and induces the overexpression of antioxidant enzymes (21). Research suggests that MLT's role in memory formation involves its influence on hippocampal neurons (22). In AD, there is a reduction in the expression of MT1 and MT2 receptors (19). Moreover, GPR50, an orphan receptor related to MLT, primarily modulates the function of MT1 receptors (15). For instance, the heterodimerization of GPR50 with MT1 receptors inhibits the MLT receptor pathway (Figure 2) (16).
MLT also has the capacity to bind to intracellular proteins, including calmodulin and orphan nuclear receptors, in order to initiate its effects on target cells (23). Moreover, MLT inhibits lipid peroxidation and shields cells from damage caused by oxidative stress (24). Previous studies have established a close relationship between oxidative stress and the onset and progression of AD, potentially through the augmentation of Aβ peptides and the intracellular accumulation of hyperphosphorylated τ protein in neurofibrillary tangles (25). MLT exhibits a neuroprotective quality, believed to be associated with its antioxidative characteristics (Figure 2). In essence, MLT not only scavenges free radicals but also promotes the expression of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), heme oxygenase-1 (HO-1), glutathione peroxidase (GPx), glutathione reductase (GRd), and γ-glutamyl-cysteine synthase (γ-GCS) (26).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a regulatory protein and transcription factor that controls oxidative stress and the transcription of antioxidant enzymes. It has been suggested that MLT, by inducing NRF2, can exert its cytoprotective and antioxidant effects. Neurodegenerative diseases like AD are linked to reduced NRF2 levels in the brain (27). Substantial evidence underscores the role of the Nrf2 signaling pathway in mediating MLT's therapeutic and biological effects (28).
3. The Role of Melatonin in Memory and Learning
A recent study demonstrated that MLT was effective in ameliorating spatial learning and memory deficits caused by exposure to isoflurane. These beneficial effects were associated with a reduction in neuroinflammation and neuroapoptosis (29). Another investigation indicated that MLT injections could mitigate neurological abnormalities in mice, potentially by modulating the N-methyl-D-aspartate (NMDA) receptor (30). Additionally, a recent study suggested that MLT might provide protection against hippocampal damage and memory deficits in cerebral hypoperfusion rats by downregulating calcium-activated potassium channels (31). In an experiment, the time spent in a specific quadrant and the time required to locate a platform were significantly reduced following the administration of exogenous MLT. It has been noted that MLT plays a role in regulating the reproduction and differentiation of neural stem cells (32), a function that supports the survival of new neurons (33). Furthermore, MLT serves as an internal regulator of neurogenesis in AD patients (34).
Neurogenesis plays a crucial role in memory formation, cellular plasticity, and cognitive health. However, the aging process leads to neurogenesis dysfunction, increasing the vulnerability of neurons to AD (14, 35). Cholinesterase inhibitors promote the survival of newly generated neurons and boost neurogenesis in adult mice (36). In this context, MLT exhibits anti-amnesic effects by reducing acetylcholinesterase (AChE) activity (37). AD is closely associated with neurogenesis impairment (38). The decline in neural progenitor cell proliferation and MLT secretion during aging and AD suggests that MLT regulates neurogenesis in the adult brain (39). This regulatory role of MLT in neurogenesis is supported by both in vitro and in vivo studies. MLT enhances the survival, proliferation, and differentiation of neural stem cells (NSCs) (32, 40, 41). It also promotes dendrite growth through MLT receptor-mediated signaling pathways involving CaMKII and CREB (38, 42).
Furthermore, MLT's impact on cognitive dysfunction observed in AD is mediated through microRNA (miRNA) signaling pathways. MiRNAs are small, single-stranded, non-coding RNA molecules that repress gene expression by binding to mRNA targets with complementarity. Studies have shown that MLT exerts beneficial effects on memory deficits in AD patients through the miR-124 pathway (43). Additionally, in rats with memory impairment induced by systemic administration of scopolamine (mimicking AD-like cognitive deficits), MLT treatment induces changes in the miR-124 pathway, improving spatial memory and mitigating long-term potentiation (LTP) impairment (43). Considering MLT's influence on various molecular signaling pathways in AD, it merits consideration as a therapeutic approach in AD treatment.
4. Therapeutic Potential of Melatonin in AD
Decreased fluctuations in blood MLT levels, which become more pronounced from middle age onwards, can lead to a decline in various aspects of nervous system function (44). MLT has been confirmed to possess anti-inflammatory, antioxidant, anti-fibrillogenic, anti-hyperphosphorylation, and anti-amyloidogenic properties (38), suggesting that it may play a protective role in neurological diseases (39). While several meta-analyses have examined the effectiveness of MLT in cognitive improvement (40, 45), numerous studies have reported inconsistent results regarding MLT's efficacy in treating AD (41, 42).
The potential link between MLT and AD development became apparent when it was discovered that reduced plasma MLT levels are associated with AD. AD patients have lower MLT levels in their cerebrospinal fluid (CSF) compared to individuals in the same age group (29). Some studies have sought to explain MLT's effectiveness by its impact on sleep disturbances in AD patients (40). For instance, exogenous MLT treatment improves symptoms of REM sleep behavior disorder (RBD) in AD patients. RBD is a parasomnia characterized by abnormal behavior or excessive motor activity during sleep (46). Overall, MLT supplementation, especially in long-term treatment, positively affects sleep quality and alleviates sleep disturbances reported by AD patients (47). Given that short duration and poor-quality sleep are risk factors for AD (40), MLT supplementation may potentially slow AD progression by enhancing both sleep duration and quality (48).
Furthermore, MLT has a favorable impact on emotional function, making it a valuable asset in delaying AD onset if mild cognitive impairment (MCI) is detected early and preventive measures are taken (8). Research has also demonstrated that MLT is effective in improving emotional and cognitive functions in both AD and MCI patients (49). It may play a significant role in AD pathogenesis by implementing preventive measures that slow down MCI progression (43), ultimately aiding in the improvement of memory and cognitive problems in these patients (50).
The effectiveness of MLT in animal models of AD has been extensively investigated. In behavioral studies using AD mouse models, MLT has been shown to enhance performance in tasks such as novel object recognition and passive avoidance (51). Additionally, research has demonstrated improvements in long-term recognition task performance in young, middle-aged, and elderly MLT-deficient mice (52). Labban et al. examined the potential prophylactic effects of MLT against spatial memory deficits in a sporadic mouse model of AD induced by D-galactose and aluminum chloride (52). In a study conducted by Corpas et al., chronic administration of MLT was found to enhance learning and memory abilities in mice, increase exploratory behavior, and reduce anxiety associated with AD in 12-month-old mice (53).
Other studies have revealed that long-term oral administration of MLT promotes synaptic growth in the hippocampus and preserves the neuronal and glial structure in a sporadic rat model of AD (54). Furthermore, animal experiments have shown that MLT can prevent the formation and accumulation of Aβ plaques and neurofibrillary tangles (55). Findings from studies in transgenic animals suggest that it is crucial to administer MLT in the early stages of AD to regulate Aβ metabolism and primarily prevent Aβ formation (56). Some research has indicated that MLT may reduce the expression of beta-site APP cleaving enzyme 1 (BACE1), the major beta-secretase involved in the generation of Aβ peptides, as well as p-tau and Aβ1-42 in AD mice (51). Additionally, documented evidence shows that MLT reduces tau hyper-phosphorylation and inhibits apoptosis in various AD model studies (29).
5. Mechanisms of MLT Improving Effects on AD
The levels of MLT increase, reaching their peak around puberty, and gradually decline with age in the elderly (57, 58). This decline has been associated with age-related neurodegenerative diseases, including AD. Indeed, the progression of AD-related neuropathology, coupled with a reduction in MLT levels in cerebrospinal fluid (CSF), makes brain cells more susceptible to oxidative damage. MLT possesses strong free radical scavenging (59) and anti-amyloidogenic (60) properties. In postmortem, AD brains, an increase in the production of free radicals, lipid peroxidation, oxidative damage to proteins and DNA, as well as a decrease in ATP production and cell viability, have been observed compared to age-matched healthy controls (61, 62).
Interestingly, it has been reported that similar to the day-night MLT rhythm, Aβ production also follows a circadian pattern, with lower levels during sleep compared to wakefulness (63). Aβ production leads to the oxidation of proteins, DNA, and lipids due to the production of free radicals. MLT, by targeting the Nrf2, down-regulates prooxidant enzymes and up-regulates antioxidant enzymes, thereby reducing oxidative damage to the brain (64). The balance between Aβ production and clearance plays a crucial role in AD progression. MLT, through receptor-dependent mechanisms and the deactivation of the JNK/Sp1 pathway, inhibits JNK phosphorylation, consequently reducing Sp1-DNA binding activity. Sp1 activates the transcription of BACE1 and APP, which promotes amyloidogenesis. Therefore, the effect of MLT shifts the balance towards increased Aβ clearance (65).
Cholinergic dysfunction is a significant contributor to the initiation and progression of AD (66). The impaired learning and memory observed in AD patients are associated with the loss of cholinergic neurotransmission and a decrease in ACh levels (Hampel et al.) (67). Impaired spatial learning and memory in a mouse model of scopolamine-induced amnesia improved with MLT treatments, as they restored the hippocampal and septum cholinergic system. This was evident through the elevation of levels of choline acetyltransferase, high-affinity choline transporter, vesicular acetylcholine transporter, and muscarinic acetylcholine receptor M1 (M1R) under MLT treatments (68). MLT reversed the impairment in recognition memory and passive avoidance performance in a D-galactose/AlCl3 AD mouse model, as indicated by the increase in prefrontal cortex AChE levels and BDNF/CREB1 expression (69). Moreover, MLT administration prevented the elevation of AChE activity in a rat model of AD (70). A study on the scopolamine model of cognitive impairment found a correlation between memory loss and elevated AChE activity. They discovered that MLT-treated animals had lower levels of AChE activity and malondialdehyde (71).
Neurovascular dysfunction is another pathological issue in AD patients (72, 73). Impaired Aβ clearance leads to Aβ deposits around cerebral vessels, disrupting blood-brain-barrier (BBB) permeability and vascular function (73). In this pathological condition, glucose transporters downregulate brain microvessels, resulting in neuronal metabolic dysfunction that correlates with tau hyperphosphorylation (74) and Aβ aggregation (75). Additionally, it has been shown that vascular endothelial growth factor (VEGF), a growth factor for vascular endothelial cells, and VEGF receptors upregulate AD disease in response to Aβ (76, 77), indicating that VEGF is involved in Aβ-induced vascular changes. MLT can ameliorate Aβ-induced vascular alterations by regulating VEGF signaling (78).
6. Conclusions
Clinical studies have revealed alterations in MLT secretion in AD, and MLT treatment appears to be effective in managing both the psychological and pathological symptoms of AD. MLT improves sleep duration and quality, memory, and cognitive issues. It also prevents the formation of Aβ plaques and neurofibrillary tangles, reduces tau hyperphosphorylation, minimizes oxidative damage, and inhibits apoptotic pathways in animal models of AD. Furthermore, MLT has been shown to modulate neurogenesis, a process significantly implicated in AD. Overall, MLT appears to be of critical importance in the treatment of AD, given its multifactorial nature, and it can be considered as one of the drug therapies for AD treatment.