1. Background
Neurodegenerative diseases are characterized by the progressive dysfunction of synapses, neurons, glial cells, and their networks (1). Neurodegenerative diseases are promising targets for stem cell-based therapies since the progressive loss of both neurons and glial cells inevitably leads to irreversible damage to the central and peripheral nervous system (2). Optimization protocols to increase the efficacy of stem cell therapy have been tested in animal models with promising results. It seems that the use of safe and potent inducers of stem cell differentiation into neuronal progenitor cells is useful in cell therapy of systemic destructive diseases. One of the most accessible and effective sources of stem cells for treating neurological disorders is neural stem cells; however, they are difficult to obtain from adults (3). It has been shown that injected BMSCs can differentiate into neurons in vivo to improve neurological behavior (4, 5). Because BMSCs can rapidly proliferate and differentiate into neuron-like cells under certain conditions, their potential application for the treatment of nerve injury and degeneration is of ongoing interest (6).
Studies have shown that selegiline, as an irreversible inhibitor of monoamine oxidase, is effective in differentiating stem cells into neuron-like cells by changing the expression of certain genes and proteins in the cell (7). Selegiline, a monoamine oxidase (MAO) inhibitor, was developed by Zoltan, the Hungarian pharmaceutical company Chinoin. In 1989, the US Food and Drug Administration (FDA) approved selegiline for the treatment of Parkinson’s disease. Later, in 2006, the FDA also approved the transdermal form of the drug (8). Selegiline has been shown to increase the expression of nerve growth factor and glial-derived neurotrophic factor in the nigrostriatal and mesolimbic dopamine signaling pathways (9, 10). In addition, selegiline may have neuroprotective effects and may slow the progression of Parkinson’s disease by promoting the production of neurotrophins such as brain-derived neurotrophic factor, nerve growth factor, and glial cell neurotrophic factor. These neurotrophins play an important role in protecting neurons from the inflammatory process. The induction and activation of multiple stress antioxidants and anti-apoptotic factors by selegiline may contribute to the maintenance of healthy brain tissue (11). As a neuroprotective drug, it can induce bone marrow stromal cells and differentiate them into neuron-like and glial cells in vitro (12). In addition, statins, such as lovastatin, are potent cholesterol-lowering agents used clinically in cardiovascular events associated with atherosclerosis (13). In addition, its safety has been confirmed in vivo and in vitro. Statins have pleiotropic effects, including antioxidant, immunomodulatory, and neuroprotective properties (14-16). Interestingly, previous data have shown that statins can alter the expression of neurotrophic factors in various animal models of neurological disorders (17).
2. Objectives
Considering that stem cells are used for the regeneration and treatment of various diseases, this study aims to determine the synergistic effect of lovastatin and selegiline on the differentiation of bone marrow mesenchymal into neural progenitor cells.
3. Methods
3.1. Isolation and Expansion of Bone Marrow Mesenchymal Cells
The ethics board of Zanjan University of Medical Sciences approved all experimental protocols (IR.ZUMS.REC.1400.243). Bone marrow mesenchymal cells were harvested from 28-day-old rats under deep anesthesia and cultured in 25 cm2-adherent flasks in Dulbecco’s modified Eagle’s low-glucose medium (DMEM; Sigma-Aldrich) with 10% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin and 100 mg/mL streptomycin (Sigma-Aldrich). The isolated cells were incubated at 37°C in 5% CO2 for 2 days. Adherent cells were harvested and subcultured, and the culture medium was changed every 2 days until the cells were 70 - 80% confluent. Cells were harvested with trypsin-EDTA (0.25%; Sigma-Aldrich) and passaged 3 times (P3).
3.2. Experimental Groups
Cells were assigned to different experimental groups as follows: Control (BMSCs without induction), experiment-1 (BMSCs induced with 20 µM selegiline for 24 hours), experiment-2 (BMSCs induced with 6 µM lovastatin for 24 hours according to our previous study (18)), and experiment-3 (BMSCs induced with 20 µM selegiline for 24 hours and 6 µM lovastatin for the next 24 hours).
3.3. Real-time Polymerase Chain Reaction
Total RNA was extracted using a pure link RNA mini kit (Invitrogen) according to the company’s instructions. 1000 ng of purified RNA extracted from cultured cells was used to synthesize 20 μL of cDNA according to the RevertAid™ Reverse Transcriptase kit (Fermentas, Germany). Nestin and NF-68 mRNA levels were determined using cDNA. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control for normalization. RT-qPCR was performed with the primers shown in Table 1. The primers were designed using Gene Runner software (3.05) and prepared by the distributor (Gene Fanavaran Co., Iran). The PCR solution contained forward and reverse primers (200 nM each), cDNA (0.5 μL), SYBR® Green I (6.5 μL; Fermentas; Thermo Fisher Scientific, Inc.), and nuclease-free water to a final volume of 12.5 μL. The PCR reaction was repeated for 40 cycles, each cycle consisting of 15 seconds at 95°C followed by 1 minute at 60°C. The relative expression of each gene was calculated using the ΔΔCt method with Pffal efficiency correction (19).
| Gene | Accession # | Sense 5 → 3 | Antisense 5 → 3 | bp | Tm |
|---|---|---|---|---|---|
| nestin | NM_001308239 | CAAATCTGGGAACTGGTAGAG | CCTAGAGCCTTCAGTGTTTC | 149 | 59.97 |
| Nf-68 | NM_031783 | ATATGCAGAATGCCGAAGAG | CTTCGATCTCCAGGGTCTTA | 147 | 60 |
| GAPDH | NM_017008 | GCCTCCAAGGAGTAAGAAAC | GTCTGGGATGGAATTGTGAG | 141 | 60 |
a In addition, the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an internal control. The table also lists the gene’s NCBI accession number, sense and antisense primers for each gene, fragment size (bp), and melting temperature (Tm). Primers were designed using Gene Runner 3.05 software (Gene Fanavaran Co., Iran).
3.4. Statistical Analysis
Statistical analysis was performed using SPSS version 15 software. All data are presented in triplicate independent experiments with mean, standard error of the mean. One-way ANOVA and Tukey’s post hoc test were used for data comparison between groups. Data with a p-value less than 0.05 were considered significant.
4. Results
4.1. Primary Cell Culture and Neuronal Differentiation
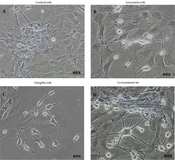
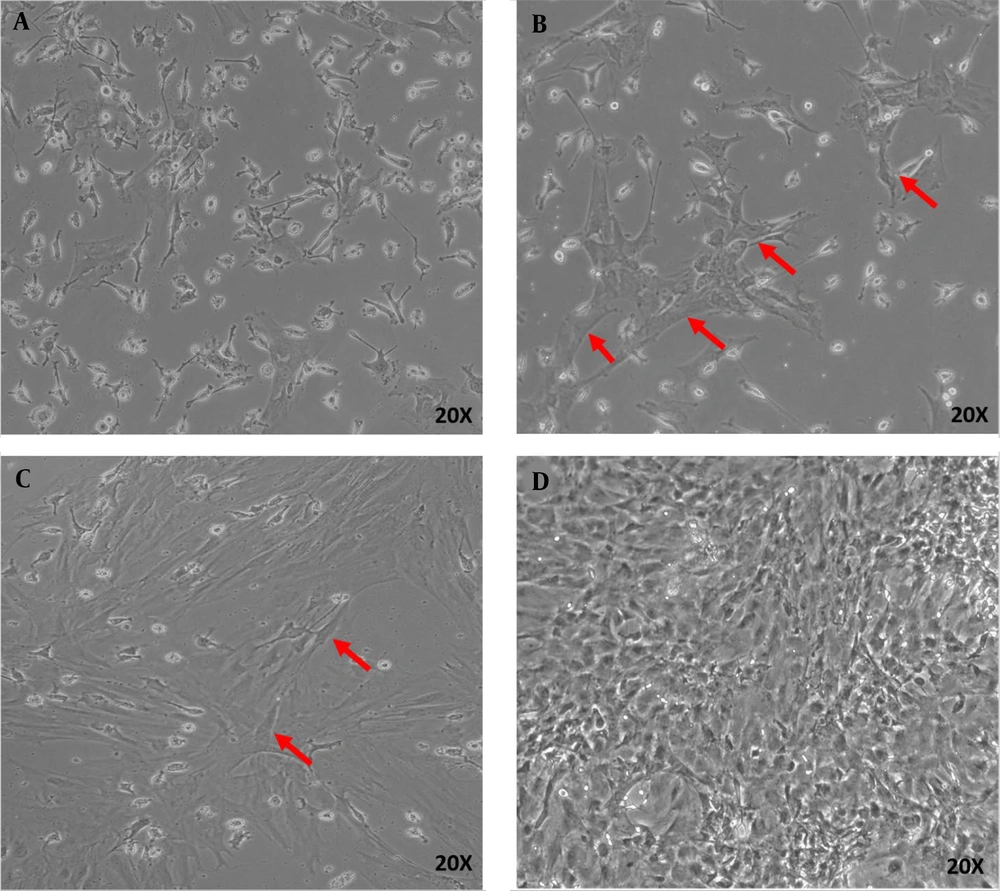
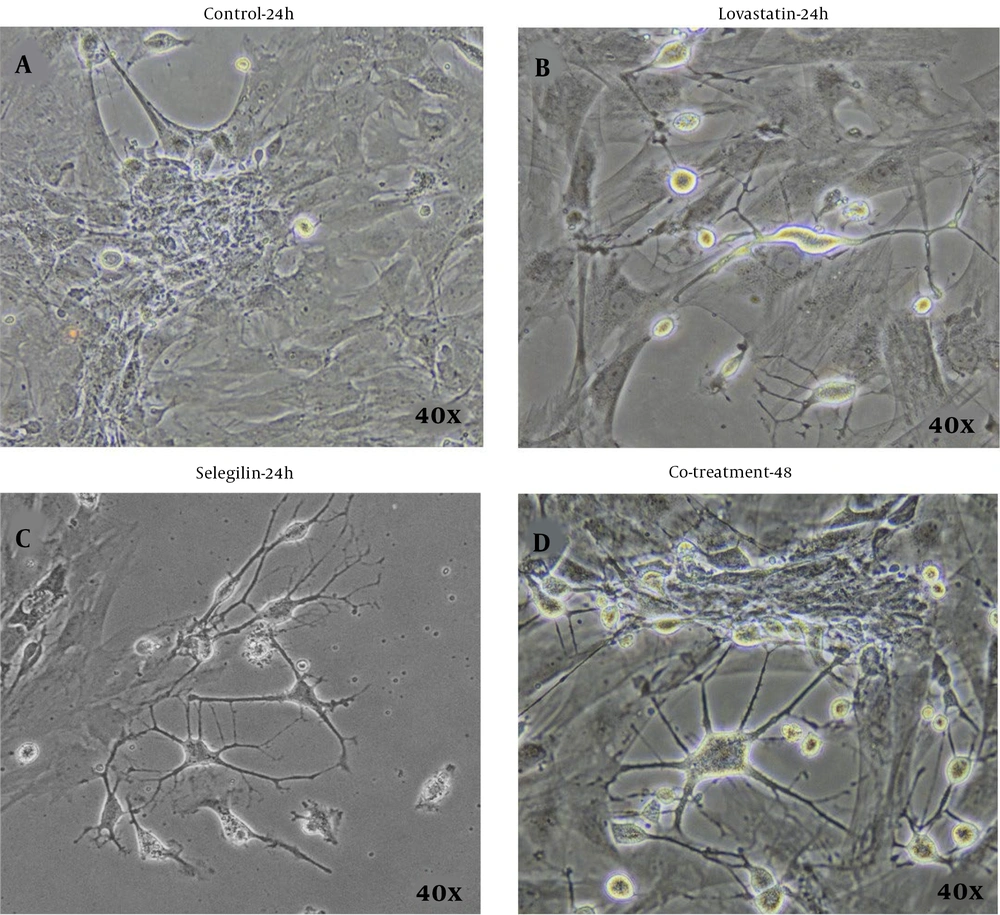
The primary culture of isolated BMSCs showed that the cells attached to the bottom of the culture plate were characterized by rapid proliferation (Figure 1A - D). In the first hours of culture, the cells floated. Their rounded nuclei were visible (Figure 1A). After 24 h, the floating cells attached themselves to the plate to form fibroblast-like colonies (Figure 1B). The first step occurred after 7 days when the cells reached 80% confluence (Figure 1C). After three passages were completed within 18 days, the cells were used for differentiation experiments (Figure 1D). Then, BMSCs were cultured in 20 μM selegiline for 24 hours. The resulting cells were positive for the nestin and NF-68 genes (Figure 2). The results showed the presence of selegiline cell bodies and a large number of neurites with a neuronal phenotype. The results are shown in Figure 2A - D. In experimental group 2 (BMSCs induced with 6 μM lovastatin for 24 h), changes in the appearance and number of cells with a neural phenotype were less than in other experimental groups (Figure 2C). In experimental group 3, cells had longer neurites and larger soma than in other experimental groups (Figure 2D).
4.2. Gene Expression
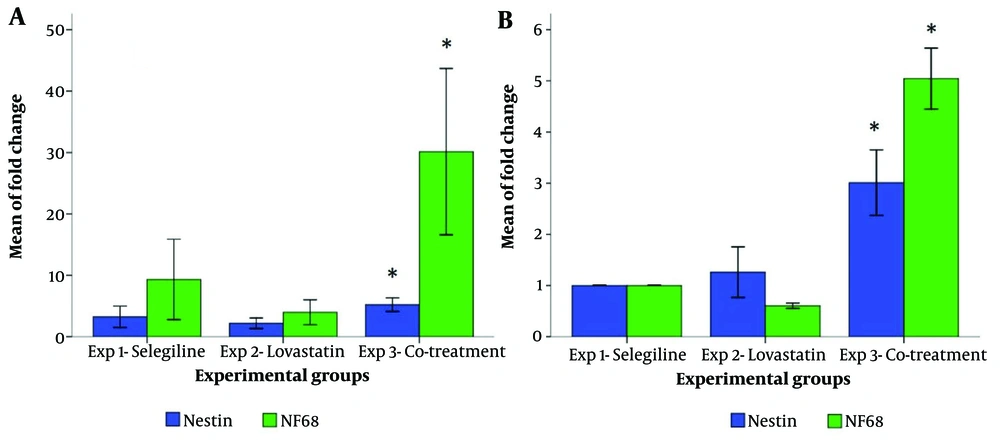
Changes in nestin expression and NF-68 mRNA levels in the experimental groups were examined using real-time quantitative RT-PCR. Figure 3A shows the results relative to the control (BMSCs without induction). In the group treated with 20 μM selegiline for 24 h and 6 μM lovastatin for the following 24 h (experiment 3), nestin (5.22 ± 0.43) and NF-68 (30.14 ± 4, 26) compared to Experiment 1 (3.24 ± 0.24, respectively 0.67 and 9.32 ± 2.54) and the group of Experiment 2 (respectively 2.18 ± 0.33 and 3.98 ± 0.79 ). Figure 3B shows the results relative to Experiment 1 (BMSCs treated with 20 μM selegiline for 24 h). In experimental group 3, the levels of nestin (3.01 ± 0.24) and NF-68 (5.04 ± 0.23) mRNA were higher compared to experimental group 2 (1.26 ± 0.19 and 0, respectively, 6 ± 0.01) increased significantly.
Real-time quantitative RT-PCR results, A, relative to the control group (BMSCs without induction) and B, relative to experimental group 1 (BMSCs induced with 20 μM selegiline for 24 h). All data were normalized to GAPDH mRNA amplification. Bars represent mean ± SEM; * (Compared to other experimental groups) P-values less than 0.05 were considered significant.
5. Discussion
The results of this study showed that biphasic induction of bone marrow stem cells by selegiline and lovastatin significantly increased nestin and NF-68 gene expression. A differentiated neuronal phenotype also occurs in the presence of selegiline. Mesenchymal stem cells are an attractive source of regenerative therapies due to their ability to perform immunomodulatory, anti-inflammatory, and non-tumorogenic functions (20). Recently, cell and molecular studies revealed interesting properties of selegiline, opening new possibilities for neuroprotective mechanisms and a disease-modifying effect of MAO-B inhibitors (21). Neurofilaments (NFs) are major components of the axonal cytoskeleton and are composed of three subunits: NF light chain (NFL), medium chain, and heavy chain. When axons are injured, NFs are released into the extracellular space and ultimately into the CSF and blood. Elevated levels of them can be used as a biomarker of nerve damage (22). Bone marrow mesenchymal cells are an attractive source of cell therapy, and their healing properties have been confirmed in animal models of neurological diseases such as stroke, Parkinson’s disease, and spinal cord injury (23, 24). Methods that stimulate the differentiation of BMSCs into functional neurons need to be developed. Bone marrow mesenchymal cells can be easily obtained and expanded in culture and promote modest functional recovery after transplantation into animal models with injured or degenerative CNS (25). The use of pharmacological agents that are less toxic but more effective in the cell culture environment is a very important point in the differentiation of stem cells into neural progenitor cells. According to studies on the effects of statins on the protection and repair of damage to the nervous system, the use of statins in a culture medium together with the active substance selegiline suggests a potentially improved mechanism.
According to in vitro and in vivo studies, selegiline prevents MAO-B and improves the synthesis of neurotrophic factors (26, 27). It is also an anti-Parkinson’s drug with antioxidant and antiapoptotic properties (28). However, its cytoprotective mechanism is still not fully understood (29). The results obtained from this study indicate a potential strategy for optimizing the use of stem cells in the treatment of nervous system injuries, including spinal cord injuries. As an irreversible MAO-B inhibitor, selegiline has antiapoptotic and neuroprotective effects (30). It also induces stem cells into neuronal and glial lineages by altering gene expression (7). Reports have shown that when used as a cell inducer, selegiline increases cell survival compared to other inducers, such as dimethyl sulfoxide. It also induces cells with morphological changes and expresses nestin genes as markers of neural progenitor cells (31). The results of this study showed that selegiline-induced BMSCs induced nestin and NF-68 gene expression. Other studies have also shown that selegiline, as a neuroprotective drug, can induce neuron-like cells that express NF-68, synapsin-1, and nerve growth factors (32). During the last few years, the Neurofilament-light chain (NF-L) has been shown to be a valuable biomarker for several neurodegenerative diseases (33). The neurofilament light chain is a neurofilament subunit highly expressed in axons and dendrites, where it contributes to structural stability in neurons. Neuroaxonal damage due to inflammatory, neurodegenerative, traumatic, or vascular injury results in the release of large amounts of NfL (33).
In addition, Mardani et al. showed that treatment of adipose stem cells with selegiline can induce these cells in neural progenitor cells expressing nestin and NF-68 (34). Nestin develops as neuronal progenitors in neuroepithelial cells and immature astrocytes of the central nervous system. In the adult brain, nestin is expressed in neurons and progenitor cells and can be expressed in reactive astrocytes under pathophysiological conditions. In this study, these markers were used to identify neuron-like cells (35). Statins are drugs approved by the Food and Drug Administration (FDA) to lower cholesterol and are widely used in clinical practice. Recently, statins have been recognized to have a variety of effects, including anti-inflammatory, antioxidant, and neuroprotective (36). In vivo experiments have shown that statin treatment after brain injury increases synaptogenesis and neurogenesis without changing blood cholesterol levels. Statins are also thought to induce neuroprotection by releasing neurotrophic factors and inducing gene expression (37, 38). Previous studies have reported that statins (atorvastatin) reduce inflammation and neuronal apoptosis after spinal cord injury and significantly improve motor recovery in rats (39, 40). It has also been reported that treatment of rat hippocampal neural stem cells with lovastatin results in increased NGF expression (41). In this study, we used selegiline as a pre-inducer and lovastatin as an inducer to differentiate BMSCs into neuronal cells expressing the NF-68 gene. Experimental data from numerous animal and cell culture studies have shown that selegiline protects against various neurotoxins, reduces oxidative stress, and has neurotrophic and anti-apoptotic effects. All of these properties may contribute to its neuroprotective activity (42)
Both drugs are safe and used in medical clinics for therapeutic purposes. In this study, considering the role of neurofilament light (NFL) in the maturation and structural support of neurons, the increased expression of this gene may indicate the role of the synergistic effect of selegiline and lovastatin in the induction of neuronal stem cells. NFL has emerged as an important filamentous protein in neurodegenerative diseases. NFL is a structural protein that forms neurofilaments, fills the axonal cytoplasm, and regulates synaptic transmission and organelle transport (43). Reported that developing mouse motor neurons express high levels of NFL (44). Studies have shown that this type of filament plays an important role in postsynaptic termination and influences nerve transmission, contributing to normal synaptic function and neuropsychiatric disorders (22). Therefore, the results of this study may make the use of lovastatin promising in clinical trials for the treatment of neurodegenerative diseases.
5.1. Conclusions
Based on the increased expression of nestin and NF-68 genes, the presence of lovastatin has a synergistic effect on neuronal differentiation and optimization of stem cell therapy approaches.