1. Context
A significant portion of the global population, particularly in developing countries, suffers from protein deficiency. Animal protein plays a crucial role in human nutrition, offering health benefits, cost efficiency, and production efficacy. The quality and quantity of animal protein should ideally meet certain standards. Global statistics suggest that individuals should ideally consume 25 - 30 grams of animal protein per day. However, in Iran, the average daily consumption is only 22 grams, which is 30% below the recommended intake. Chicken meat stands out for its low conversion rate, minimal investment and space requirements, and lower production costs compared to other sources of animal protein. Consequently, the breeding of broiler chickens has seen significant advancements to meet the increasing demand for meat worldwide. Currently, chicken meat production heavily relies on the introduction of foreign strains. Given the widespread popularity of chicken meat in the global diet, finding a suitable substitute is challenging. The primary objective of genetic projects is to identify sequence variations associated with key economic traits in chickens (1). This review explores the efficacy of Candidate Genes (CGs) and various molecular markers in relation to economic traits in chickens.
2. Evidence Acquisition
2.1. Use of Candidate Genes
Candidate Genes are among the principal molecular methods used to identify specific genes linked to economic traits in chickens. Numerous genes have been identified with clear associations with production and reproductive traits. For example, Zhao et al. found that the Uncoupling Protein (UCP) gene reduces metabolic efficiency in organisms (2). Similarly, Sharma et al. observed an association between the UCP gene and growth traits in commercial chickens (3). Polymorphisms in the TGF-β3 gene were linked to growth and body composition traits in broilers and Leghorn chickens. Additionally, the insulin-like growth factor 1 (IGF1) gene was identified as important for the growth of various chicken strains (4). Liver FABP1 acts as a key regulator of liver fat metabolism and participates in beta-lipid oxidation. Other researchers have reported that this gene can be considered an indicator of heat tolerance (5). The BMPR-IB gene significantly affects egg production in broiler lines, while the ODC gene plays a crucial role in biological processes such as differentiation and apoptosis, leading to accelerated growth and egg production in chicken lines. Using CGs, strains can be quickly segregated based on economic traits during the selection process. However, a drawback of this method is the lack of prior information on physiological, biochemical, or functional pathways, such as hormonal regulation and biochemical metabolism pathways, which are essential for understanding complex and quantitative traits. These pathways are often limited or unavailable.
2.2. Application of Molecular Genetics in Chicken Breeding
In previous years, poultry breeding was primarily based on phenotypic traits such as egg number, body weight, and egg weight. Unfortunately, these traits are influenced by environmental factors, posing challenges to the enhancement of superior genetic stocks. However, recent advancements in biochemical techniques have enabled scientists to directly access the genetic code, providing a means to select superior organisms without the influence of environmental factors. The consensus linkage map was initially published in 2000 (6), marking a significant milestone in this field. Subsequently, numerous efforts have been dedicated to understanding quantitative trait loci (QTL) in chickens.
Many economic traits in chickens, such as growth rate and conversion ratio, are polygenic, influenced by multiple physiological factors. This complexity makes it challenging to identify a single marker associated with a gene. These loci are referred to as quantitative traits. Genetic markers linked to QTLs allow for direct selection based on genotype (7). Microsatellite markers (SSRs) have proven to be valuable tools for identifying markers associated with QTL in various livestock species, including chickens (8).
Simple sequence repeats (SSRs) exhibit high polymorphism and can effectively distinguish between closely related organisms. In chickens, SSRs are utilized to map reference populations and facilitate quantitative genetics and precision mapping approaches. For instance, Gao YuShi et al. (9) demonstrated the feasibility of SSR fingerprinting in analyzing genetic relationships among Chinese domestic chicken breeds. Additionally, the genetic diversity of domestic Turkish chicken breeds was assessed using 10 SSR markers (4). Simple sequence repeats are instrumental in designing genetic diversity research and conservation strategies.
Currently, DNA fingerprinting technology is extensively employed to assess genetic variation and relatedness in diverse poultry and plant populations (10-12). Incorporating DNA-based SSR markers that enable sex identification into such analyses can provide highly polymorphic patterns. However, to ensure accessibility, rapidity, and affordability in genotyping individuals in chicken line populations, markers with high resolution are essential (13, 14).
Despite the utility of SSRs, a lack of these markers has been observed on micro-chromosomes, which are likely rich in coding sequences. Consequently, other genetic markers are utilized at the chicken genome level to investigate genetic relationships and assess diversity.
2.3. Development of Markers Using Next Generation Sequencing
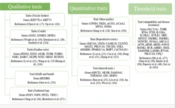
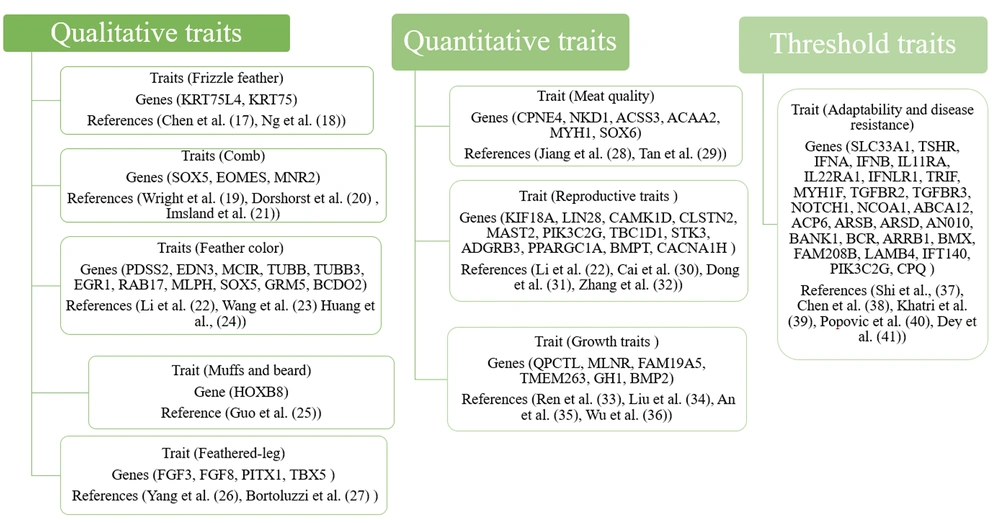
The recent advancements in molecular genetics through next-generation sequencing have created new avenues for enhancing broiler and layer yields. Next-generation sequencing offers valuable resources for developing molecular markers and designing primers for PCR and fingerprinting reactions. Utilizing markers across the entire genome is crucial for identifying potentially harmful homozygosity and preventing the loss of genetic diversity in chickens. A previous study employed Whole Genome Sequencing (WGS) on 54 native and commercial strains (15), revealing higher genetic diversity in native strains (Lari, Khazak, and Marandi) compared to the commercial Leghorn line. Another investigation employed WGS on 185 broiler chickens in China (6) to determine genetic diversity and population structure, confirming 24 introgressions of commercial meat breeds (16). Whole Genome Sequencing proves to be a cost-effective tool for generating large datasets to accurately estimate phylogenetic relationships between populations. In this study, three types of traits were investigated using WGS: Qualitative, quantitative, and threshold traits (see Figure 1).
2.4. Structural Variants
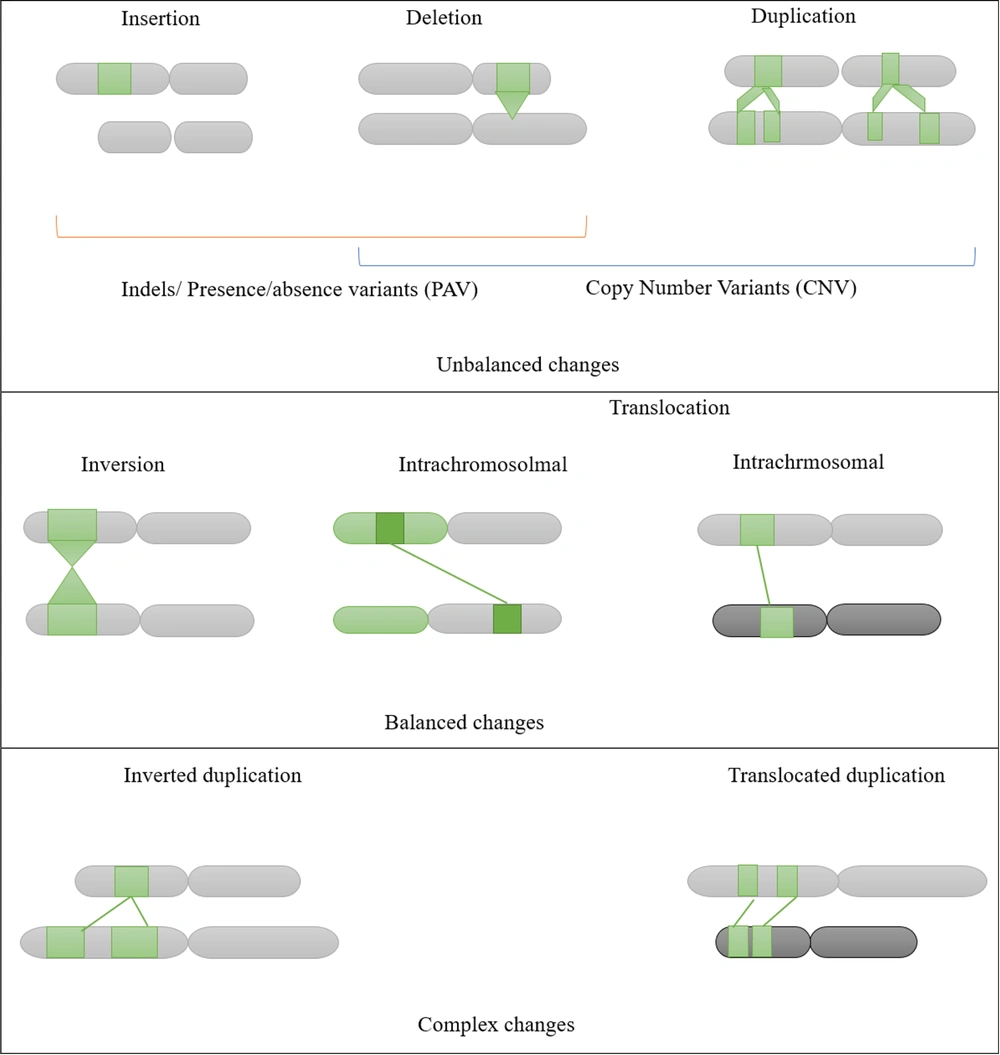
Structural Variants (SVs) are significant determinants of animal phenotypic diversity and play a crucial role in genetic diversity in livestock. Structural Variants encompass a wide range of genomic modifications, categorized into intervals: < 50bp, 50bp to 1Kb, 1 - 10Kb, 10 - 100Kb, > 100Kb, or Unknown (see Figure 2). Most SVs are associated with body size and weight, plumage coloration, and pigmentation in chickens (42). These mutations are grouped into two classes: Unbalanced modifications, which alter DNA content, and balanced modifications, such as inversions and inter or intra-chromosomal translocations, which influence the orientation and/or location of DNA. Recently, SV markers have been widely employed to determine genetic diversity and phenotypic differences in chickens.
2.5. Indels
Indels represent the primary source of molecular-level changes and are extensively utilized as molecular markers in the study of economic traits in livestock. These di-allelic markers are distributed throughout the chicken genome. Yan et al. (43) identified two indels associated with economic traits, revealing that these indels emerged during a rigorous selection process. Some functionally significant genes were found to be shared, suggesting that merging them could help elucidate the relationship between genes and traits. Chang et al. (44) identified the C2CD3 gene as the causative gene of the talpid2 mutation in chickens. Furthermore, their study shed light on the distribution of indels in the chicken genome and their potential impact on gene function, deepening our understanding of chicken genome diversity. Despite the sequencing of the chicken genome, some micro-chromosomes exhibit lower quality. Previous reports identified 158.98 MB of new sequences, including 1 335 protein-coding genes not found in the GRCg6a reference genome, through chicken Pan-genome (PG) construction. These genes play crucial roles in various biological processes, such as immune pathways. Li et al. (45) conducted PG analysis of 20 assembled chicken genomes and identified 1335 new genes involved in fatty acid metabolism, steroid synthesis, and immune response.
2.6. Copy Number Variations
Utilizing whole-genome information is essential for understanding and maintaining the genetic foundation of commercial chickens. Several studies have employed WGS in chickens, sheep, ducks, and quails. Almeida et al. (46) conducted WGS of 28 broilers, identifying approximately 9 914 904 SNPs and 793 603 indels. Similarly, Huang et al. (24) utilized various markers, including SNPs, SVs, copy number variations, and indels, to explore chicken genetic diversity. Copy Number Variations markers are also valuable for investigating complex diseases and economically important traits. Genotyping of 554 chickens from Xinghua and White Recessive Rock breeds revealed 1 875 CNVs, with approximately 109 of them being novel (47). In a study on skeletal muscle growth within the same population, polymorphic CNVs overlapping with the SOX6 gene were positively correlated with SOX6 gene expression. Using a complete F2 chicken population (n = 554) derived from a cross between Xinghua and White Recessive Rock chickens, researchers identified a total of 1875 CNVs, averaging 3.42 per individual.
2.7. Presence/Absence Variation
Presence/Absence Variations (PAV) are present in one genome but entirely absent in another. Presence/Absence Variation polymorphisms may arise from the insertion or deletion of transposable elements, simple sequence replication slippage, or unequal crossover events (48). These polymorphisms are widespread among different species and have significant evolutionary consequences.
2.8. Pan-Genomics
Current genotyping methods, such as WGS, follow the core strategy of aligning short reads to a reference genome derived from a single individual. This approach typically results in compressed haploid representations of diploid genomes or chimeric haploblocks due to allele mixing. While these methods have been successful in identifying SNPs and indels in populations, they can suffer from reference bias and may underestimate various types of structural variants (49). The use of large-scale long-read re-sequencing has the potential to mitigate some of these limitations; however, it comes with high costs and lower accuracy compared to short reads, particularly in chicken genomics. For example, while there are over 40 000 short read experiments in the Sequence Read Archive (SRA), there are fewer than 500 long read experiments in databases. Consequently, the widespread use of long reads for re-sequencing surveys in the near future seems unlikely. Previous studies have attempted to address these challenges by improving algorithms for SV detection using inexpensive short read data. However, these methods often suffer from high false positive and false negative rates (2, 47). To overcome these limitations, the use of PG as a reference has been developed (50, 51). Pan-genome contains sequences common to all individuals, along with information about the position, alleles, and frequencies of each variant site within the input assemblies. The PG of domestic chickens was first published in 2020. By utilizing PG, a survey of 268 WGS data in chickens identified 15 205 (76.32%) core genes and 4 738 variable genes. The IGF2BP1 promoter region on chromosome 27 was found to be primarily associated with chicken growth traits. Another study reported the detection of 159 Mb of new sequences, including 335 protein-coding genes and 3 011 long noncoding RNAs, using the PG method (45). These insights into the genetic structure of diverse broilers and layers have been facilitated by chicken PG, revealing relationships between phenotypes and genes.
3. Conclusions
In this article, we have discussed molecular genetic tools for detecting economic traits in chickens. We provided a summary of CGs and molecular markers related to economic traits. Based on our findings, pan-genomics emerges as a novel approach to understanding the relationships between genetics and phenotypes in chickens. Furthermore, the pan-genomics technique has identified new genes related to important biological pathways and has improved the quality of the reference genome.